Improve Your Cooling Tower Treatment
Cooling water plays an essential role in process operations at many plants. For a majority of these facilities, the cooling system contains one or more cooling towers. The metals used in the tower, cooling water piping and heat exchangers may include carbon steel, galvanized steel, copper alloys and stainless steel. Protecting all these metals from corrosion and minimizing scaling and microbiological fouling in cooling systems pose ongoing challenges. Adding to the difficulty, operators face emerging restrictions on the discharge of a number of impurities in cooling tower blowdown, including phosphorus, zinc and other metals, biocide residuals, and dissolved and suspended solids. This article examines evolving chemistry that provides improved corrosion and fouling protection while also being more environmentally friendly than previous treatment methods.
Microbiological Fouling
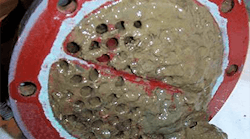
This often is the most problematic and severe issue in cooling water systems (Figure 1). Even with fresh water as the makeup source, such fouling can occur rapidly. The risk has gotten even worse at many plants that now have switched to alternative, lower quality water, e.g., secondary wastewater plant effluent, for plant makeup. Such supplies potentially can introduce large quantities both of nutrients (nitrogen species and phosphorus) and food (organic compounds) to cooling water, thereby increasing the potential for fouling.
For decades, plants have used some form of oxidizing biocide as the core treatment for microbiological control. Gaseous chlorine once was the common choice due to low cost but safety concerns influenced movement toward safer compounds, at first liquid bleach. However, many makeup water supplies are mildly alkaline, and the most common cooling tower treatment programs for the last four decades have relied on alkaline chemicals — and alkaline conditions can render chlorine compounds somewhat ineffective. Consider the reaction when chlorine is first injected into water:
Cl2 + H2O ⟺ HOClaq + HClaq (1)
Hypochlorous acid, HOCl, is the killing agent. As pH rises, HOCl increasingly dissociates:
HOClaq ⟺ H+aq OCl-aq(2)
The hypochlorite ion, OCl-, is a much less powerful killing agent than HOCl.
Figure 1. Severe fouling can afflict a heat exchanger (left) and tower fill (right) in a cooling water system.
A common enhancement to this chemistry came from blending bleach and a bromine compound, usually sodium bromide (NaBr), to produce HOBr, the bromine analog to hypochlorous acid. It is an effective oxidizer and dissociation occurs at a higher pH than HOCl, producing more of the effective undissociated acid. Now, though, other alternatives are becoming more popular due to their ease of application and improved ability to kill microorganisms.
A key aspect of any program is to treat the water before organisms can settle on cooling system surfaces. Organisms that attach and multiply rapidly will form a slime layer for protection and, within this film, numerous microbial colonies will develop, including anaerobic bacteria like the sulfate reducers whose metabolic byproducts can induce severe corrosion (Figure 2).
Compounds such as monochloramine (NH2Cl) and monobromamine (NH2Br) have exhibited strong results in attacking settled microbiological colonies. Their efficiency appears due to the compounds being weaker oxidizers than chlorine or bromine; they aren’t consumed by the slime layer but rather penetrate to the microorganisms underneath to kill them. Production of the compounds must occur in situ by mixing bleach, dilution water and ammonium salts just prior to injection.
Meanwhile, hydantoins, a group of compounds known for a long time, now are being employed in new ways. Hydantoins are ring structures; a common product is 1-bromo-3-chloro-5,5-dimethylhydantoin.
Figure 2. Metabolic byproducts of anaerobic bacteria such as sulfate reducers can cause pitting.
When this compound dissolves in water, it releases chlorine and bromine that then serve as the biocide. The halogenated hydantoins typically are manufactured as granules or tablets. These commonly are loaded into a compartment connected to a side-stream loop on the cooling water system. The product slowly dissolves and is introduced to the main cooling water.
New chemistries have been developed where non-halogenated hydantoin molecules are in a liquid solution and are introduced separately from an oxidizer. The hydantoin then serves to stabilize the oxidizer and improve residence time in the cooling water. In contrast to ammonium salts that can react vigorously if contacted with bleach without adequate dilution, you can safely blend liquid hydantoin with bleach in the injection line. These formulations may include a biodispersant to break apart the protective slime layer to allow the biocide to better contact the underlying organisms.
Plant personnel often seem to overlook the use of non-oxidizing biocides to supplement the oxidizing compounds. These non-oxidizing products function either by damaging the cell wall of organisms or interfering with metabolic processes within the cells. Table 1 lists properties of some of the most common non-oxidizers.
Non-oxidizers are more persistent and typically are shot fed to the cooling system only two-to-three times per week in conjunction with oxidizers. Due to their persistence, they are more effective in dead legs, closed cooling systems and under layup conditions. However, you must carefully evaluate the microbial species in the cooling water to determine the most effective non-oxidizing biocides because their action targets specific metabolic functions. Always apply antimicrobial compounds, whether oxidizing or non-oxidizing, in accordance with the directions on the label. Where discharged to “waters of the state,” you must incorporate them into the plant’s National Pollutant Discharge Elimination System (NPDES) permit. Also, as with all chemicals, safety is an absolutely critical issue when handling biocides.
Corrosion And Scale Control
A critical aspect of proper water treatment equipment design and chemistry program selection is obtaining comprehensive and, ideally, historical raw water quality data. Often, plant owners and technical personnel underestimate the importance of complete raw water data and prepare project specifications with an incomplete water analysis (see: “Don’t Foul Up Your Water Treatment Program”). Raw waters typically contain many impurities, a number of which can combine to form deposits or influence corrosion. Table 2 lists several common impurities and their potential consequences.
Additional deposition of silicate, sulfate and fluoride salts, and other compounds also is possible.
For the last four decades, the core of the most common cooling tower treatment method has been inorganic and organic phosphate (also known as phosphonate) chemistry to control both scale formation and corrosion. Supplemental zinc addition provided further corrosion protection. However, new developments are leading to a significant change.
Phosphate/phosphonate programs emerged as the preferred technology with the phase-out of chromate-based corrosion inhibitors in the 1980s due to environmental concerns. The chemistry can be effective but is much more complex than chromate treatment. Upsets in chemical feed or control, or changes in makeup water chemistry may lead to deposition and corrosion. Now, another factor —increasing restriction on discharge of phosphorus and zinc in cooling tower blowdown — is propelling a transition in treatment methods. Many areas of the country are controlling, at least from point sources, discharge of phosphorus to the environment because it’s the limiting nutrient for toxic algae blooms (Figure 3), such as the severe on-going ones in Florida.
Figure 3. Phosphorus in cooling water blowdown is a key nutrient for blooms such as this one. Source: Jesse Allen and Robert Simmon, NASA Earth Observatory.
New non-phosphorus/non-zinc programs not only eliminate such discharges but also can provide an additional benefit. Some new chemistries, e.g., reactive polyhydroxy starch inhibitors (RPSIs) such as FlexPro, offer less potential for fouling and much better corrosion protection than the phosphate-phosphonate programs. Unlike the phosphate-phosphonate treatments, which rely on deposition of such products as calcium phosphate, iron phosphate and zinc hydroxide for corrosion inhibition, an RPSI, per the functional groups on the molecules, actually forms a surface barrier on metals.
On a pound-per-pound basis, RPSI currently is competitive with phosphate/phosphonate treatment methods. Moreover, it significantly improves corrosion protection, more than tipping the scales in favor of the new chemistry. However, its application, like that of any other new technology, requires careful evaluation of process conditions to ensure success. The product is sensitive to excessive sulfate concentrations in the water, although it shows excellent results in protecting metal surfaces from chlorides. Some users, in trimming feed rates to perceived ideal conditions, have reduced the concentration too far, which, in turn, allowed localized corrosion sites to develop. Difficulties have arisen in cooling waters with high iron concentration (which can pose problems under many circumstances). Here, iron bacteria form black, unsightly colonies, particularly if the biocide feed is insufficient. These deposits provide a quite nasty example of the consequences of inadequate microbiological control. Another important issue is the location of corrosion coupons for monitoring product performance. In general, corrosion rates increase with increasing temperature. Corrosion coupons placed in cool locations of the system may not accurately indicate performance at other spots, particularly the surfaces of heat exchanger tubes. Also, systems that previously have suffered corrosion and rusting likely will require a significantly higher dosage than necessary with new, clean systems.
Keep Cool
Many plants appreciate the need for proper treatment of cooling water. After all, fouling, scaling and corrosion in a cooling water system can cost a site millions of dollars in lost heat transfer and equipment damage. Today, additional factors such as the environmental impact of makeup and discharge chemistry heighten the stakes even more. So, facilities should keep up-to-date on treatment options and reconsider their programs.
BRAD BUECKER is a senior technical publicist for ChemTreat, Glen Allen, Va. RAYMOND M. POST, P.E., is the director of cooling technology for ChemTreat, Glen Allen, Va. Email them at [email protected] and [email protected].