Twin breakthroughs by researchers at King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, overcome two issues holding back development of microbial electrosynthesis (MES) technology.
[pullquote]
MES involves using electrical currents to stimulate microorganisms into manufacturing useful chemical products.
The KAUST researchers are studying chemolithoautotrophs, microbes that typically reside in caves, hydrothermal vents and other locations where energy sources such as sunlight and organic carbon are in short supply, if they exist at all.
The microbes consume CO2 through their natural metabolism, creating small organic molecules as a byproduct. It’s this ability that Pascal Saikaly, an associate professor of environmental engineering at KAUST, and his team have harvested.
“The microbes obtain their energy from the oxidation of inorganic compounds, such as hydrogen, iron and sulfur,” explains Bin Bian, a PhD student from Saikaly’s team. The microbes strip the inorganic compounds of electrons while taking up CO2 and reducing it to organic products as part of the process.
The idea itself isn’t new; indeed, many research projects have focused on MES. However, the typical MES reactor uses chemolithoautotrophs grown on a submerged flat-sheet cathode, with CO2 then bubbled into the solution. According to team member Manal Alqahtani, this has two key problems: flat-sheet cathodes are difficult to scale up, and CO2 gas has poor solubility.
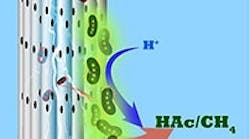
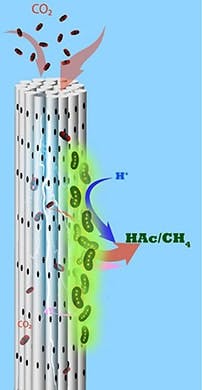
So, the team developed an alternative MES reactor using cathodes made from stackable, cylindrical porous nickel fibers that Saikaly’s group previously applied to recover water and energy from wastewater. Here, CO2 is pumped through each cylinder, and electrons flow along it.
“Using this architecture, we directly deliver CO2 gas to chemolithoautotrophs through the pores in the hollow fibers,” Alqahtani explains. “We provided electrons and CO2 simultaneously to chemolithoautotrophs on the cathode surface.”
In Alqahtani’s initial study, the porous nickel hollow fibers act as an inorganic electrocatalyst for hydrogen generation from proton reduction and as a gas‐transfer membrane for direct CO2 delivery to CO2‐fixing hydrogenotrophic methanogens (biological catalysts) on the cathode. These novel features create a suitable environment for the enrichment of methanogens, which utilize the hydrogen as a source of reducing equivalents for converting CO2 to methane.
Caption: Microbes growing on porous cylindrical electrodes suck in CO2 and turn it into useful chemicals such as acetate and methane. Source: Bin Bian/KAUST.
The microbes were able to convert CO2 to methane with 77% efficiency, compared to 3% efficiency with a conventional MES design.
The team attributes the hybrid bioinorganic system’s performance to the electrode architecture, which provides a three‐phase boundary for gas-liquid reactions, with the reactions supported by both the inorganic and biological catalysts.
A follow-up study looked to improve performance further by coating the electrodes with carbon nanotubes.
The thinking here is that to improve both biofilm formation on the cathode and product generation rates, the cathode design needs a high specific surface area and enhanced electrode-microbe electron transfer. This study aimed to demonstrate a novel cathode design made of porous nickel hollow fibers (Ni-PHFs). The pores in these hollow fibers facilitate direct delivery of CO2 to the bacteria Sporomusa ovata in MES.
Modifying the surface of Ni-PHFs with carbon nanotubes (CNTs) resulted in an 11-fold increase in the CO2 adsorption capability at atmospheric pressure, as well as 76.3% reduction of cathode electron transfer resistance.
According to the team, this cathode surface modification partially explained the higher acetate production rate of 247 ± 17 mM/d/m2 from direct CO2 delivery through the pores of the Ni-PHF/CNT cathode, compared to 145 ± 4 mMd/m2 for the Ni-PHF cathode. Higher electron recovery in the form of acetate (∼83%) was also observed for the Ni-PHF/CNT cathode. As for the tests where CO2 was sparged into the medium, acetate production was 36% lower than tests with direct CO2 delivery to S. ovata through the pores of the Ni-PHF/CNT cathode.
These results demonstrate that using the PHF electrode design and modifying the cathode morphology to enhance the microbe-electrode interactions and CO2 availability for bacterial growth are effective approaches to increase the rates of CO2 reduction in MES, the team notes.
“So, the nanotubes enhanced the electron transfer from electrode to chemolithoautotrophs and in tests using acetate-producing microbes, production of the chemical almost doubled when the nanotube coating was applied,” emphasizes Bian.
Meanwhile Alqahtani’s ongoing work includes investigating easier approaches to develop porous cylindrical cathodes, while Bian is optimizing CO2 flow rates and investing renewable MES energy sources, such as solar.
Seán Ottewell is Chemical Processing's Editor at Large. You can email him at [email protected].