Cooling towers serve a crucial role at many chemical plants but often receive only passing attention. However, cooling system fouling, scaling and corrosion can incur enormous costs related to materials replacement and from efficiency degradation and perhaps even lost production. Fortunately, cooling tower water-treatment technologies and programs have evolved significantly over the last decade, in large part due to more-stringent regulations regarding discharge and choice of makeup water supplies.
Today, plants generally can take steps to effectively combat fouling, scaling and corrosion. Let’s look at how to address these issues.
Microbial Fouling
An obvious but challenging aspect of cooling tower operation is keeping the tower, condenser and other system components free of microbiological fouling, scaling and solids deposition. Even with fresh water from a lake or river as makeup, cooling system chemistry control requires diligence and good planning.
Cooling systems provide an ideal environment — warm and wet — for microbial colony formation. Bacteria will grow in condensers and on cooling tower fill (Figure 1), fungi on and in cooling tower wood, and algae on wetted cooling tower components exposed to sunlight. Biocide treatment is absolutely essential to maintain cooling system performance and integrity.
Figure 1. Fill, like other cooling tower elements, offers an attractive surface for microbial growth. Source: Ref. 1.
Bacteria fall into the following three categories:
• aerobic, which utilize oxygen in the metabolic process;
• anaerobic, which live in oxygen-free environments and use other sources, i.e., sulfates, nitrates or other donors for their energy supply; and
• facultative, which can thrive in aerobic or anaerobic environments.
A problem with microbes, particularly bacteria, is that once they settle on a surface the organisms secrete a slimy polysaccharide film for protection. This film then will collect silt from the water, thus growing even thicker. Even though the bacteria at the surface may be aerobic, the slime layer allows anaerobic bacteria underneath to flourish. These organisms in turn can generate acids and other harmful compounds that directly attack the metal. Microbial deposits also establish concentration cells, where the lack of oxygen underneath the deposit causes the locations to become anodic to other areas of exposed metal. This often results in pitting, which can cause tube failure well before the expected lifetime of the material.
Fungi will attack cooling tower wood in an irreversible manner, which can eventually lead to structural failure. Algae will foul cooling tower spray decks, potentially reducing performance and creating unsafe working locations. (See: “Enter a Tower with Caution”).
Algae are also a food source for amoeba and other protozoans, which in turn are important in the growth and amplification of airborne pathogens, such as Legionella pneumophila, the bacteria that cause Legionnaires disease [2].
The core of most microbiological treatment programs is an oxidizing biocide to kill organisms before they can settle on heat exchanger tube surfaces, cooling tower fill and other locations. Chlorine was the workhorse for many years; adding gaseous chlorine to water causes the following reaction:
Cl2 + H2O ⇔ HOCl + HCl
Hypochlorous acid (HOCl) is the killing agent, as it oxidizes internal cell components and disrupts the organism’s metabolic processes. Due to safety concerns with gaseous chlorine, many plants switched to bleach (sodium hypochlorite, NaOCl) as the oxidizing biocide. And most recently, reliable systems have been developed that generate chlorine on-site via electrolysis of salt solutions. These use ordinary salt, softened water and electricity to produce a dilute solution of hypochlorite, which is safer than storing and handling large quantities of sodium hypochlorite [3].
Regardless of the source of chlorine, pH greatly affects the functionality and killing power of the chemicals due to the equilibrium nature of HOCl in water:
HOCl ⇔ H+ + OCl-
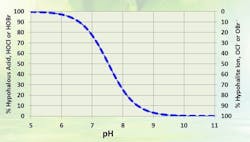
OCl- is a much weaker biocide than HOCl, probably because the charge on the OCl- ion doesn’t allow it to penetrate cell walls. The dissociation of HOCl rapidly increases as the pH rises above neutral (Figure 2). Thus, for common alkaline-scale/corrosion-treatment programs, chlorine chemistry may not work well.
Figure 2. As pH increases, treatment effectiveness decreases because less HOCl is in solution. Source: Ref. 1.
Ammonia or amines in the water further compromise performance by reacting irreversibly with chlorine to form much less potent chloramines. This is becoming more of a factor because both existing and especially new plants — by choice or mandate — increasingly are moving away from fresh water for makeup and relying upon alternative sources. A prime example is use of secondary wastewater from a publicly owned treatment works. This water often contains significant concentrations of ammonia, phosphorus and organics. If fed directly to the cooling tower, water containing these microbiological nutrients and food can totally disrupt any chemical treatment. Technologies such as membrane bioreactors, moving bed bioreactors and others are becoming more common as pretreatment methods for makeup water [4]. (For details on some specific developments, see: “Wastewater Treatment Gets a New Spin.”)
Chlorine Alternatives
A popular replacement for chlorine has been bromine chemistry, where chlorine or bleach and a bromide salt, typically sodium bromide (NaBr), are blended in a makeup water slipstream and injected into the cooling water. The chemistry produces hypobromous acid (HOBr), which has similar killing powers to HOCl but dissociates at a higher pH. However, guidelines for discharge of wastewater, including cooling tower blowdown, are becoming more stringent, often due to actions taken by individual states above and beyond U.S. Environmental Protection Agency mandates. Rumors continue to circulate that future regulations will address bromide. Such actions could curtail bromine chemistry.
Chlorine dioxide (ClO2) has achieved some popularity. The pH doesn’t affect its killing power and, unlike chlorine, the chemical doesn’t react with ammonia and doesn’t form halogenated organic compounds. Also, ClO2 is more effective in attacking established biodeposits.
Unfortunately, ClO2 is unstable and must be generated on-site. The additional complexity, hazards, and costs of chlorine dioxide generation must be weighed against the benefits.
All of the powerful oxidizers mentioned above may be ineffective if microorganisms are allowed to settle and establish the protective slime layer. The chemicals are consumed by the slime without killing the organisms underneath. So, it is imperative that biocide feed systems be operated and maintained properly to control colony formation. If colonies do form, it may be necessary to establish at least temporary feed of a biodispersant [2, 5]. A biodispersant, similar to a detergent, helps penetrate and disrupt biofilms to destroy the organisms underneath.
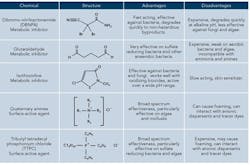
One method to help control microbes is to supplement the oxidizing biocide with regular application of a non-oxidizing biocide, perhaps once or twice per week. These antimicrobials are more specific in their mode of action than oxidizing biocides and are often classified as either metabolic inhibitors or surface active agents depending on their primary more of action. Table 1 highlights the pros and cons of the most common non-oxidizers.
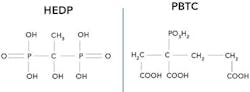
Figure 3. Many treatment programs now rely on either 1-hydroxyethylidene-1,1-disphosphonic acid (HEDP) or 2-phosphono-butane-1,2,4-tricarboxylic acid (PBTC).
Careful evaluation of the microbial species in the cooling water is necessary to determine the most effective microbial control program. All of the products should be used in strict accordance with the directions on the label. They must be identified and addressed in the plant’s National Pollutant Discharge Elimination System (NPDES) permit if the cooling water is directly discharged.
As with all chemicals, safety is an absolutely critical issue when storing and handling antimicrobial compounds. Adherence to all handling guidelines and use of proper personal protective equipment is a must. Many of these chemicals will attack human cells as well as those of microbes and the oxidizing compounds often are highly reactive with other materials.
Scale Control
In the vast majority of cases, the first scale-forming compound that will appear in cooling systems, particularly heat exchangers, is calcium carbonate (CaCO3). Calcium and bicarbonate alkalinity in solution drop out due to inverse solubility with temperature:
Ca2+ + 2HCO3- + heat → CaCO3 + CO2 + H2O
In the “good old days” of cooling tower operation, a very common treatment program consisted of a first step of sulfuric acid addition to the makeup to remove bicarbonate alkalinity:
H+ + HCO3- → CO2↑ + H2O
A typical pH range of the circulating water was 6.5 to 7.0 or thereabouts, which minimized scale formation. Treatment with sodium chromate provided corrosion protection by causing the steel surface to form a pseudo-stainless steel layer. However, chromate treatment in all open-recirculating and most other cooling water systems has been banned for years due to the toxicity of hexavalent chromium (Cr+6). The popular replacement has been alkaline-based treatment, primarily relying on inorganic and organic phosphates (phosphonates), with a supplemental polymer to sequester and modify non-carbonate scale-formers. Figure 3 shows the structure of two common phosphonates.
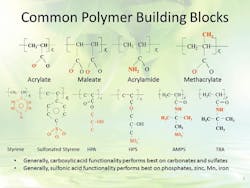
These formulations typically include inorganic ortho- and poly-phosphates and perhaps a low dosage of a zinc salt to assist with corrosion control of steel. Advanced polymeric dispersants are used to maintain the phosphate and zinc corrosion inhibitors in solution at the concentrations required to control corrosion, and to prevent them from depositing on heat transfer surfaces. These polymers may have a variety of active groups that principally act by crystal modification and dispersion (Figure 4).
However, phosphate is a prime nutrient for plant growth and many receiving bodies of water have been designated as “phosphorus impaired” because they suffer from toxic algae blooms. Moreover, these effects may increase the chlorine demand in cooling systems and can cause fouling in the critical high temperature heat exchangers prevalent in the chemical industry. Thus, the major water treatment chemical companies have been furiously developing non-phosphorus and non-zinc cooling water treatment programs.
Figure 4. All-polymer treatment programs contain one or more active groups, chosen based on the cooling water’s constituents. Source: Ref. 1.
The best programs today significantly outperform their phosphate and zinc predecessors in terms of passive film formation, fouling resistance, and in the ability to protect stainless steel from pitting and stress corrosion cracking.
Along with new chemicals have come much improved technologies to feed and monitor them. Key to this progress has been development of on-line sensors, communications hardware, and trending software that enable product concentrations and performance to be continuously tracked and reported. Sophisticated chemical feed systems monitor the product concentration and provide fresh chemical only as needed.
Save Cold Cash
The careful choice of cooling water treatment technologies and program can avoid problems in your cooling tower system. This will cut maintenance requirements and improve operations, adding significantly to your plant’s bottom line. However, even an effective program still requires regular inspection of the cooling towers.
Get Additional Details
The pre-workshop seminar of the 37th Annual Electric Utility Chemistry Workshop in Champaign, Ill., on June 6–8, 2017, will delve more into the subject. For information about this event, go to: www.conferences.illinois.edu/eucw.
BRAD BUECKER is senior process specialist with Kiewit Engineering Group, Inc., Lenexa, Ks. RAYMOND M. POST, P.E., is the director of cooling technology for ChemTreat, Glen Allen, Va. E-mail them at [email protected] and [email protected].
REFERENCES
1. Post, R. and Buecker, B., “Power Plant Cooling Water Fundamentals,” pre-workshop seminar for 33rd Annual Electric Utility Chemistry Workshop, Champaign, Ill. (June 13, 2013).
2. “Legionellosis Guideline: Best Practices for Control of Legionella,” WTP-148, Cooling Technology Institute, Houston (July, 2008).
3. “Miox Acquisition Rounds Out Johnson Matthey Water Purification Business,” Chemical Processing (June 16, 2016).
4. Buecker, B., Willersdorf, W. and Shulder, S., “Cutting Edge Concepts for Makeup Water Production,” pre-workshop seminar for the 36th Annual Electric Utility Chemistry Workshop, Champaign, Ill. (June 6, 2016).
5. Boudreaux, K., “Effects of Balance of Plant Systems on Overall Heat Rate,” 2017 Spring Meeting, Power Plant & Environmental Chemistry Research Committee, EPRI-sponsored (April 10–12, 2017).
6. Kalakodimi, P. and Post, R., “Benefits of No