THIS MONTH’S PUZZLER
Our decanter for separating a mixture of C10, C20, C30 and C40 compounds from a water/toluene solution isn’t performing right. To save space, we opted for a 3-ft-diameter vertical unit with a length/diameter ratio of about 3:1. The flows are 6,000 pounds per hour (PPH) of organic and 2,500 PPH of aqueous phase. The viscosities are: 300 cP for the organic; 0.8 cP for the water. The specific gravities are: 0.8 for the organic; 0.99 for the water. Feed is at about 50% of the straight side length and we use a dispersion layer of about 5%. Although the decanter is designed to capture 100 micron (µ) droplets, the carbon filter that’s supposed to serve as a water polishing filter actually is removing them. We’ve had to change it out every 30 hours at a cost of $250/500 pounds. On examination, we discovered that during construction 1-in. high-flow packing was dumped into the decanter. It fills the bottom third of the column. How can we fix the decanter? Should we be happy with 100 µ? How can we reduce our carbon costs?
RE-EXAMINE THE DESIGN
I would do the following to resolve the problem. Remove the packing from the decanter as it is taking up valuable space. And install a dual basket filter in the feed line to break the organic/water mixture and facilitate the phase separation in the decanter. Such filters are commercially available.
Review your design against the following suggested method. These gravity-based decanters are based on hydraulic balance and have been successfully used for continuous processes.
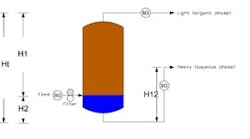
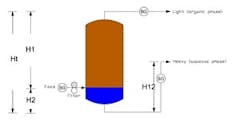
The density numbers suggest that we should have a quick separation of the organic and water phases. Based on your problem definition, if the nozzle for removal of the organic layer is located at about seven feet from the top straight side, there is sufficient residence time to separate the two layers. Actual time can be determined in the laboratory. The feed location as described should be all right. The following equation can be used to calculate the height of the interface:
H2 = (H12 - Ht (ρ1/ρ2))/(1 - (ρ1/ρ2))
where ρ1 is the light phase density;ρ2 is the heavy phase density; Ht is the distance between the exits, light and heavy; H12 is the height of the riser in the heavy-phase exit line; and H2 is the height of the heavy phase (see Figure 1).
The top of the tank and the water outlet pipes should be connected in such a way that they equalize the pressure so that the liquid does not siphon out. The water outlet pipe should have an adjustable arm so that the height of the water layer can be adjusted. It can be used to control the interface level.
Don’t use the manual shut-off valves in the water and organic outlet pipes to adjust flow and interface layer height.
Girish Malhotra, president
EPCOT International, Pepper Pike, Ohio
The 1 in. packing at the bottom of the decanter is to facilitate following actions:
1) intercept and collect individual oil droplets; 2) combine several small droplets into larger ones; 3) allow rising of the enlarged oil droplets by gravity.
Correctly designed and maintained system should easily capture 100 micron
droplets. Since you are facing problem, check for any chocking/plugging of packing. If the packing is dirty it may require cleaning [It may not be conditioned properly -- plastic, metal and ceramic packing needs to be conditioned to remove its factory coating.] Another idea is to install a fine filter upstream of decanter to remove any solids. A 6-in. thick knitted wire mesh pad installed near the water outlet nozzle may also improve separation. [Plan carefully for removal for cleaning.]
CCS Reddy, lead process design engineer
Singapore Refining Company, Singapore, Indonesia
CHANGE THE CONTINUOUS PHASE
The viscosities are very different and, in this application, the water should be the continuous phase and the organic should be the dispersed droplet phase. I would check this first. Because of the relative amounts of the two phases (70% organic), the organic is likely to be the continuous phase. Look at the process forming the emulsion.
If the feed emulsion cannot be modified, perhaps water could be recycled and added to the feed, making water the continuous phase. The decanter can give superior separation; even at the higher total inlet feed rates needed for a system with a recycle.
Look at the inlet and outlet nozzles. The inlet velocity may be too high and may encourage stirring and mixing in the dispersion band. If necessary, add a diffuser internal to the decanter. Consider the packing.
The packing in the bottom of the decanter is serving as a static mixer, decreasing separation performance. Removing of the packing should be beneficial.
Lastly, the temperature is not discussed as a variable. Running the decanter at a warmer temperature may also improve separation.
Mike Gentilcore, principal research engineer
Covidien Imaging Solutions, Hazelwood, Mo.
REDUCE SURFACE TENSION
Dump the column, remove the packing. The current mechanical design won’t work. Instead, design an impingement plate for the entrance to help divide the two streams in the dispersion zone. If the feed pump permits, install a static mixer at the inlet to the decanter.
The process design for the vertical decanter is even less promising. Although the residence time for a 3-ft-diameter decanter is more than sufficient to remove the droplets, the calculated entrainment droplet size is about 2,000 μ. This is not the 100 μ desired and explains why carbon is being rapidly consumed. Carbon columns usually capture droplets in the range of about 30 μ.
There is another reason for reducing the overuse of the activated carbon — disposal: roughly $0.50/lb for transportation (within 200 miles) and $0.60/lb for disposal by drying and incineration via a thermal oxidizer (TOX). Once you factor in the disposal, for this example you’re paying $1.60/lb as the true cost of carbon. A more realistic price for activated carbon is about $1.80/lb, including shipping to the site. The final price would then be $2.90/lb. Regeneration is possible and co-generation, typical of a TOX, might reduce costs. Let’s reconsider the design again.
With decanters sometimes efficiency can be improved by increasing the diameter or the temperature. However, a quick check with these parameters shows no improvement: the viscosity of the continuous phase, i.e., the light phase, is too high. We need to consider other options.
If you can add a small trace element, perhaps a surfactant that will not contaminate the organic phase or can be separated easily, this might be the direction to go. Anything that reduces the surface tension may be effective.
Do a drain test in the lab. Cut a circular hole in a small container with about 100 ml of the organic phase. The hole should be smooth but sharp, without a nozzle. Measure the time it takes to drain through the hole after adding the chemical or doing whatever you decide to do. You’ll want to run a standard first with the untreated organic. If you’re making headway, the drain time should decrease.
You must reduce the surface tension of the dispersed light phase. Reducing the aqueous phase by half will cause a phase inversion but this trick won’t improve separation of the two phases.
Dirk Willard, contract staff engineer
Hemlock Semiconductor, Hemlock, Mich.
JANUARY’S PUZZLER
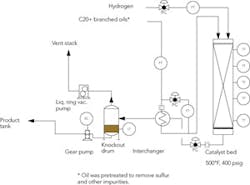
Our hydrogenation system, consisting of a column filled with catalyst, an interchanger and a knockout drum under vacuum (Figure 2), produces excess hydrogen. The drum is meant to separate the hydrogen from the product, which is then pumped to the tank farm. The vacuum pump surges constantly, unable to find a sweet spot. The pump vents to atmosphere perhaps as much as 3 std. ft3/min of hydrogen. Our plant manager wants to know if there’s a way to recover the hydrogen and stabilize the level feeding the pump. How can we address this situation? Is there any way to limp along with this process until it’s convenient to shut it down?
Send us your comments, suggestions or solutions for this question by December 10, 2010. We’ll include as many of them as possible in the January 2011 issue and all on CP.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to Process Puzzler, Chemical Processing, 555 W. Pierce Road, Suite 301, Itasca, IL 60143. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.