Johns Hopkins ChemEs Crack Crucial Chip Challenge
Key Highlights
Chemical liquid deposition creates new opportunities: Johns Hopkins researchers developed chemical liquid deposition, a scalable process using familiar equipment and aqueous solutions to create amorphous zeolitic imidazolate frameworks (aZIFs), enabling manufacturers to produce semiconductor materials with existing chemical industry infrastructure.
Packing more into smaller chips: The aZIF materials work as highly sensitive positive-tone resists that can withstand high-powered radiation needed for creating microscopic circuits invisible to the naked eye.
Dual-purpose technology: Beyond semiconductor applications, aZIFs serve double duty as gas-separation membranes for chemical processing.
Researchers at Johns Hopkins University have created electronic circuits so small that they are invisible to the naked eye, solving the chip manufacturers’ headache of how to squeeze smaller features onto their products in a way that is suitable for a production line.
Currently, manufacturers coat silicon wafers with a radiation-sensitive material known as a resist. When a beam of radiation is applied to it, a chemical reaction burns details into the wafer. However, the high-powered beams needed to carve out these ever-smaller details don’t interact strongly with traditional resists.
“While the advanced lasers required for imprinting on the miniscule formats already exist, researchers needed new materials and new processes to accommodate ever smaller microchips,” explained lead researcher Michael Tsapatsis, a Bloomberg Distinguished Professor of chemical and biomolecular engineering at Johns Hopkins.
The research team had previously shown that new resists made of amorphous zeolitic imidazolate frameworks (aZIFs) could withstand the higher-powered radiation sources being tested.
Working with Swiss, Chinese and other U.S. research organizations, the group developed a new methodology called chemical liquid deposition (CLD). Using this, researchers can study various combinations of aZIFs easily and quickly, according to a Sept. 11 article in Nature Chemical Engineering.
The CLD Breakthrough
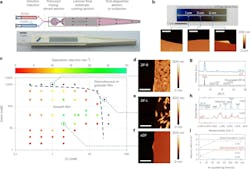
At the heart of the process is a 3D-printed reactor with a well-stirred mixing region, leading to a laminar flow coating region.
Aqueous solutions of zinc nitrate and 2-methylimidazole (2-mIm) linker are fed into the reactor separately at 2 ml/minute via a two-channel syringe pump. The two solutions merge and enter the 0.24 ml well-stirred section for vigorous mixing by a stir bar.
After a few seconds there, a moderately reactive, non-aggregated liquid mixture forms, from which smooth and continuous aZIF films can be continuously deposited by CLD under laminar flow conditions in the reactor’s second section.
The flow field in the microreactor is designed to allow the results of film depositions to be analyzed quantitatively.
To demonstrate a wider applicability of the CLD method, the researchers formed aZIF films of Zn-benzimidazole (aZIF-Zn/BIm), Zn-4,5-dichloroimidazole (aZIF-Zn/dcIm) and Co-2mIm (aZIF-Co/2mIm).
They found aZIF-Zn/BIm to be a particularly sensitive positive-tone resist. These are crucial qualities. The first means high-resolution patterning happens with lower energy than needed with traditional resists, while the second means that areas of film exposed to the electron beam become more soluble and are subsequently removed during the liquid-phase development process.
This research creates major opportunities for the chemical industry. The CLD method uses familiar equipment and aqueous solutions, making it ideal for chemical manufacturers to scale up. The aZIFs have dual applications as next-generation photoresists for semiconductor manufacturing and as gas-separation membranes for chemical processing. This positions chemical companies as essential suppliers to the semiconductor industry while also improving their own separation processes.
aZIF Evolution and Project Funding
aZIFs and their applications are the latest in a long line of technical advances made by the Tsapatsis research group. In 2009, for example, the group gave one of the first-ever demonstrations of gas separation by a metal organic framework (MOF) membrane.
In 2015, the researchers started investigating crystalline ZIFs as membranes for propane/propylene and other gas separations and three years later developed a solvent-free thin-film synthesis method based on atomic layer deposition to make them.
“At the same time, we realized that solvent-free methods also provide an avenue for aZIFs, and with support from the U.S. Department of Energy, started efforts to deposit aZIF films by molecular-layer deposition (MLD),” Tsapatsis explained.
Advanced Functional Materials published the group’s first report of aZIF film synthesis by MLD in 2024.
DOE and the National Science Foundation (NSF) are funding part of the project, and Tsapatsis said he is writing a proposal for more, so the method can be extended to other amorphous and crystalline ZIFs and MOFs.
“Although conceptually feasible, there is considerable effort required to identify deposition conditions for high-quality films when a metal and/or imidazole is substitute for another,” he said.
In May, the group won NSF funding for a $500,000, two-year project to investigate how underlying chemical reactions associated with the new process help it avoid the unwanted and indiscriminate physical sputtering effects associated with traditional plasma-based etching processes.
In September, the group received another $3.54 million from NSF to install and establish a high-tech facility to advance photoresist research in extreme ultraviolet radiation (EUV) lithography at Johns Hopkins. The facility will be available for use by scientists and engineers across the U.S. and, as well as increasing fundamental knowledge about EUV-driven chemistry, will also develop resist design principles for diverse material classes.
The next engineering challenge for the team is scaling up from 100-mm wafers used in the lab to industry standard 300-mm wafers, according to Tsapatsis.
“We will be looking for funding to start this scale up work,” he said.
Meanwhile, funding from DOE’s basic energy sciences separation program is ensuring that the membrane separation potential of the new films can be investigated, too.
Asked if any of this work had attracted industrial interest or investment, Tsapatsis replied, “Not that I can talk about.”
About the Author
Seán Ottewell
Editor-at-Large
Seán Crevan Ottewell is Chemical Processing's Editor-at-Large. Seán earned his bachelor's of science degree in biochemistry at the University of Warwick and his master's in radiation biochemistry at the University of London. He served as Science Officer with the UK Department of Environment’s Chernobyl Monitoring Unit’s Food Science Radiation Unit, London. His editorial background includes assistant editor, news editor and then editor of The Chemical Engineer, the Institution of Chemical Engineers’ twice monthly technical journal. Prior to joining Chemical Processing in 2012 he was editor of European Chemical Engineer, European Process Engineer, International Power Engineer, and European Laboratory Scientist, with Setform Limited, London.
He is based in East Mayo, Republic of Ireland, where he and his wife Suzi (a maths, biology and chemistry teacher) host guests from all over the world at their holiday cottage in East Mayo.