Process Safety: Avoid Big Trouble from Little Changes, Part 2
After defining that management of change (MoC) is applicable and after determining the needed process safety review(s) [1], the next question is what type of process hazard review is needed for the change, including for the smaller and smallest changes? What method of process hazard analysis (PHA) should be selected? As the name implies, conducting a complete and thorough process hazard review in conjunction with MoC is an essential component of an effective process safety management (PSM) program.
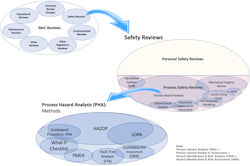
This article is part of a series focusing on hazard review requirements for small MoCs, i.e., minor modifications. These changes may involve a single component, a single flow path, or a single operating procedure step. MoC reviews involve numerous specific reviews applicable to a given change [2]. These reviews are shown in the upper left of Figure 1. The previous article in this series focused on the process safety review screening step which categorizes the change [1]. This article will show how that screening and categorization effort facilitates defining the appropriate process hazard review. Most of the discussion will explain the rationale for the methodology selection and will encourage resetting past thinking and presumptions on this topic.
Terminology for Process Hazard Reviews
Terminology varies from region to region and between regulating authorities. To clarify the terminology that will be used in this article, refer to Figure 1.
As shown in Figure 1, safety reviews are among many reviews performed under MoC [2]. Process safety reviews are a subset of safety reviews and include various types of reviews and studies such as process hazard analyses (PHAs), facility siting studies, major accident risk (MAR) studies, and others (Figure 1). PHAs may be further subdivided into the methods that are used to conduct these studies. These methods range from qualitative to semi-quantitative to quantitative methods. Specific methods may include checklist-driven studies, hazard and operability studies (HAZOP), layer of protection analyses (LOPA), quantitative risk assessment (QRA), failure modes and effects analyses (FMEA), and additional methods shown and not shown in Figure 1.
It is worth spending a few minutes discussing the specific language contained within the OSHA PSM regulation [3] because the language itself may be a source of confusion regarding allowable and appropriate process hazard review methods.
The U.S. regulation [3] separates PHA and MoC under two separate elements and chooses language that does not tie the elements together. OSHA PSM references various hazard review methods under the PHA element but refers only to “considerations. . . .” of “ . . . safety and health [3]” (i.e., a safety review) under the MoC element. Language in other elements and interpretations explicitly distinguishes PHA versus MoC hazard review requirements. These differences may be construed to mean that PHA cannot be conducted on most or all MoCs.
However, as demonstrated in Figure 1, PHA by any methodology is a subset of safety reviews. To conduct a complete MoC, all applicable reviews need to be conducted which include process hazard review using a selected methodology, such as described under the PHA element.
Likewise, some process safety industry guidance, such as Center of Chemical Process Safety (CCPS) Risk Based Process Safety [4], distinguishes between hazard identification and risk analysis (HIRA) and MoC program components. The CCPS guidance in the HIRA section explicitly describes the need for hazard review and risk analysis for projects at various stages of implementation. However, the management of change section does not provide the same emphasis and does not reference formal HIRA review technique(s) to be applied to MoC project reviews [4]. MoCs are projects involving changes of various size, scope, and complexity. As shown in Figure 1, HIRA reviews, which cover that same suite of process hazard review methodologies as PHA, also apply to MoC.
HIRA is also referred to in European standards such as COMAH [5] as hazard identification and risk assessment. These terms, PHA, HIRA, process hazard assessment, and process hazard review, are equivalent. The terms are used interchangeably in this article and refer to the same practices.
With those relationships clarified, the next question is, how do we select the process hazard analysis method for a given change or MoC?
Process Hazard Analysis (PHA) Method Selection
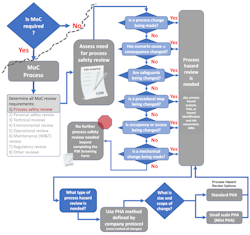
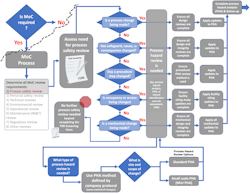
The process safety review screening tool will provide guidance on the process safety reviews needed and on the method selection [1]. As suggested here, once process hazard review has been identified as needed, the choice of process hazard analysis (PHA) method is simple. As shown in Figure 2, the decision regarding method selection is predetermined by the company PHA protocol. Choose whichever PHA method is used by the company to conduct the full facility PHA. The same methodology should be chosen to conduct all MoC process hazard reviews.
The size of the process hazard review, i.e., PHA, is dependent on the size and scope of the change (Figure 2). The size of the PHA review team and the tools that are used to facilitate the review will differ for reviews of various sizes. But the choice of method used to conduct the review can be simplified by always aligning with the company PHA protocol. That means, if the company protocol uses HAZOP and LOPA for units and processes within the facility PHA, very small changes within those units or processes can be conducted as a mini-HAZOP and a mini-LOPA study [6][7].
This simple recommendation likely differs from many operating company practices and most industry guidance. The OSHA PSM regulation itself references selecting PHA protocol dependent on the complexity of the process [3]. It is for this reason that the bulk of the remaining discussion will be devoted to the reasons why applying the same standard is a good idea.
Conducting PHA
To conduct an MoC process hazard review that meets PHA protocol requirements, the review must meet all elements of the regulatory and the company requirements. Those requirements will generally mean that:
a. The same methodology prescribed by the company protocol will be applied to all PHA reviews
b. A qualified PHA facilitator will participate in the study.
c. The PHA team will meet minimum requirements which typically include having an experienced operator and plant engineering representative present. Other specialists are brought in as needed and as applicable based on the change under review.
d. All findings are addressed via in-study closure or follow-up recommendations that are addressed and closed after the meeting.
e. Records of the PHA are retained
Many companies may view this list of requirements as impractical or unfeasible for every change. This assumption may require revisiting engrained beliefs regarding MoC and PHA practice.
Arguments for and against, . . . please explain how applying PHA to every MoC can work
“I am not buying this business about applying PHA to everything, . . . convince me . . . ”
The rationale below presents a thought process for not only why operating companies may need to rethink past MoC practices, but also why they would want to rethink those practices. Rethinking the way MoC process hazard reviews are conducted is an opportunity to streamline efforts and reduce overall cost while achieving better safety outcomes [6][8].
“The language in the Pre-startup Safety Review (PSSR) element, 1910.119 (i)(2)(iii), seems pretty clear. ‘[PSSR] shall confirm that . . . [f]or new facilities, a [PHA] has been performed . . . and [for] modified facilities . . . [MoC requirements] are complete.’ [3] The OSHA PSM regulation does not require me conduct PHA on every MoC, so why do it?”
True. The regulatory language and prior interpretations [9] indicate that PHA is not required for management of change. The regulatory language draws distinctions between safety review requirements for new versus modified facilities. These references are both explicit and implicit within the regulation and in prior interpretations. However, certain OSHA interpretations acknowledge the need for PHA updates associated with changes. The language in the June 1998 OSHA Interpretation on PHA revalidation requirements [10] makes references tying the two activities together.
. . . employers must establish the scope and extent of their PHA updates and revalidations to include at least the following: . . . checks to ensure that modifications to processes since the last PHAs have gone through management of change procedures or PHAs when required and that those changes are reflected in the PHAs; . . . [10]
The expectation that the facility PHA must “reflect” all changes that have occurred within the facility is logical given that a chain is only as strong as its weakest link. Individual changes represent individual links within the facility. Practices and protocols need to be applied consistently to ensure that the overall PHA is “accurate” [10] and has not been weakened or compromised by individual changes.
Note: The selection of the word “or” in the interpretation is curious because all changes not deemed replacement-in-kind (RIK) are required to go through MoC protocol [3]. MoC encompasses a broader set of activities beyond design and safety reviews. Those activities include document and database updates, procedure updates, training, and other activities. PHA represents a subset of many necessary reviews under MoC [1] [2]. The “or” gives the impression that activities and reviews associated MoC and PHA are somehow equivalent or alternatives to one another. This series of articles attempts to clarify the terminology and the relationships between these activities in practice and show that PHA is an embedded part of MoC rather than an alternative practice.
The screening process discussed in the prior article [1] and shown in Figures 2 and 4 addresses the “when required” condition within the interpretation. The screening step distinguishes when changes require process hazard review, i.e., PHA. [10]
“OK. But aren’t these reviews only required at the five-year revalidation? We are not being mandated by the regulation to conduct a PHA for all MoCs at the time of the MoC reviews. Isn’t that true?”
Yes, that is true. In fact, the regulatory language and interpretations allow for retroactive PHA review at the time of the five-year revalidation. However, delaying the PHA is contradictory with better practice and is inconsistent with the arguments presented in this article. Based on the definitions provided in this article, the PHA is a subset of safety reviews. Therefore, PHA is an embedded requirement and needs to be completed in conjunction with all necessary MoC reviews before MoC approval.
“But wait . . .” many may say, “ . . . even if we buy into that argument, there is no need to use full PHA protocol for every MoC, especially for those really small MoCs. Isn’t that true?”
Well, again, the U.S. OSHA PSM regulation allows for that practice. Although to “reflect” [10] the impacts of the MoC in the PHA at five-year revalidation, the standard PHA protocol must be applied. The question that practitioners should ask themselves is: Does the regulatory allowance make sense for our company from a safety, efficiency, and cost standpoint? Does this allowance make logical sense at all?
Consider analogous situations addressing other design, safety, and work practice requirements. Consider the expectations and protocol for conducting hot work, confined space entry, energy control, and welding, for example.
Would standard procedures associated with these activities be bypassed or minimized because the job was small or few people were involved? Do two sets of confined space entry or welding criteria exist based on the size of the job? Would we bypass standard requirements for energy control, hot work, or welding today on the promise that jobs would be revisited within five years to fully assess whether the work – that was already done – was acceptable?
We should hope not. Bypassing standard protocol for designing, assessing, or implementing work is considered a “stop-the-job” offense at companies with strong safety culture. Bypassing core process hazard review assessment criteria until after the work has been implemented should be a similar stop-the-job offense. Yet, this allowance has been written into the regulation, industry guidance, and accepted standard practices and procedures. Does this make sense?
Basic deductive reasoning should convince us that full PHA protocol needs to be applied to every MoC that our screening process indicates requires a process hazard review.
Despite these apparent truths, based on regulatory language and allowances as well as common accepted practices, most companies do not use full PHA protocol for all MoCs. Most companies likely do not believe that it is possible or practical to conduct fully compliant PHAs on all small or very small MoCs. These assumptions may be based on myths or misperceptions.
Given the rationale above, rather than assuming that conducting small PHAs on small MoCs is not practical, it may be worth re-examining company protocol to ensure that the procedures are aiding the company in effectively performing process hazard assessment on all changes. This re-examination may indicate a need to rewrite certain PHA protocol criteria. Alternative practices may be compliant, less costly, more effective, and lead to better safety outcomes.
Challenges and Barriers or Myths?
While some challenges exist for using full PHA protocol on MoCs of all sizes, many perceived barriers are myths. Issues that are commonly cited as obstacles to using full PHA protocol on all MoCs include:
1) A perception that all studies using full PHA protocol are lengthy, involve large teams, and will incur significant cost
2) An assumption that providing qualified PHA facilitators will be an insurmountable challenge
3) An assumption that access to PHA software is required to use full PHA protocol on all MoCs
It is a misconception that applying PHA protocol to small MoCs will be more costly and/or lengthy. Conducting MoCs to PHA protocol will not require more time or effort unless the prior MoC reviews were incomplete or inadequate. If existing MoC reviews were incomplete or superficial, there will likely be a slight increase in time and effort spent conducting a fully compliant PHA review. This impact is a good thing as we do not want process hazard reviews to be deficient or poorly performed. And these upfront investments will be recouped later through streamlined PHA revalidation efforts.
If the time and effort spent on existing MoC reviews is less than the time spent to conduct a review of the same change using PHA protocol, it is worth asking the question, why? If the company PHA protocol is sufficiently onerous that a small effective PHA cannot be conducted, it is also worth asking the question, why?
The key is applying an appropriate methodology based on the process being assessed, not based on the size of the change. For process changes or mechanical changes to systems assessed via HAZOP, the HAZOP node where the change occurred and the nodes impacted by the change require review and may require updates. If LOPA is used, the LOPA assessment for those scenarios will also require review and possible updates. For changes to procedures, a procedural PHA review is a more appropriate method [11][12]. Regardless of method prescribed by company protocol, the review effort will be scaled to the size of the change. An operations representative is always expected to be present along with any necessary technical or engineering support. With the PHA team leader, the group size may be as small as three people. The duration of a PHA review meeting for a small change may be less than an hour or two with a similar amount of time for preparation.
Supplying each review team with a qualified PHA facilitator is a genuine challenge, but if we recognize the need and reset our thinking on the topic, solutions can be found. Dumbing down the requirements for MoC hazard reviews does not solve that problem; it simply masks it.
Consider qualifying multiple tiers of PHA facilitators given the size and scope of the change. Consider supplementing external resources with qualified internal resources from the process safety group or department. Recall the advice, “Don’t DIY your MoC” [2]. Especially, don’t DIY your process hazard review. Review leaders need to be qualified process safety specialists. These changes may require upfront investment into resources, but the resource requirements on the back end, the five-year revalidation, will be reduced and the overall costs will be reduced through efficiency gains. If the PHA update process is moved to an evergreen approach, further efficiencies and gains can be realized [6].
Access to PHA software is often cited as a barrier to conducting full PHA on all changes and to moving to evergreen PHA. However, this rationale is unfounded. In the paper, The evergreen process hazard analysis [6], options are presented for conducting PHA to full company protocol for small changes which do not require access to the core PHA software. Full discussion is beyond the scope of this article. But in short, solutions can be formulated that model other existing practices, such as updating drawings. Many similarities exist. Drafting software, such as Autocad, does not reside on end users’ computers. Drawing update procedures provide work processes that allow for review, approval, and communication of changes to designated personnel that then make the revisions. Similar PHA update work processes can be created. The mark-up tools may vary depending on PHA size or complexity while still using the same assessment protocol. And newer cloud-based PHA tools can provide additional work flow options. Each company develops site-specific practices based on the available systems, tools, and resources.
Other Process Safety Reviews
Figure 4 shows a full decision support process for defining process safety review needs.
As shown in Figure 4, additional process safety studies beyond the PHA may require review or update depending on the type of change being assessed. This overview provides a high-level visual of the process. Each company, business unit, or area needs to ensure that all criteria and studies have been identified for their operations. The decision support process and the screening form should be routinely updated and continuously improved.
Conclusions
The belief that applying PHA methods to very small changes is not possible or practical is a perception rather than a reality. All PHA reviews require time and effort that is scaled to the size and scope of the change or event being assessed. PHA reviews for very small changes, e.g., mini-HAZOPs and mini-LOPAs, may be conducted by a small team in a short timeframe comparable to existing MoC hazard reviews.
The size, scope, and complexity of the change dictate the length and effort of the process hazard analysis not the method that is chosen to conduct the review. Certain tools and resources may differ for PHAs of differing sizes, but the method, the review requirements, and the deliverables should be the same.
The proper time to conduct reviews that satisfy the full extent of company PHA protocol is before, not after, the change is implemented. Redefining practices to meet this goal should result in overall cost reduction through improved efficiency. Improvements in MoC hazard review effectiveness should lead to better safety outcomes.
Acknowledgements
The author wishes to thank the 2021 Mary Kay O'Connor symposium organizers and former Chemical Processing Editor-in-Chief, Mark Rosenzweig, for their interest in this topic. The author also wishes to thank Mike Marshall, Kathy Pearson, Traci Purdum (Chemical Processing's Editor-in-Chief), and others for their review and comments on this article. The author appreciates having reviewers representing various industry perspectives.
References
[1] Olsen JE, “Process Hazard Reviews for Small Changes - Part 1: Applying sound process safety review screening to every change is the first step” pp. 19-22, Chemical Processing. February 2023.
[2] Olsen JE, “Management of Change Reviews for Small Modifications,” pp. 35-38, Chemical Engineering Progress, American Institute of Chemical Engineers, December 2022.
[3] U.S. Department of Labor Occupational Safety and Health Administration, Process safety management of highly hazardous chemicals, 29 CFR §1910.119(e) and (l), Jun 1, 1992.
[4] CCPS, Guidelines for Risk Based Process Safety, Center for Chemical Process Safety, American Institute of Chemical Engineers, New York, NY, 2011.
[5] U.K. Health and Safety Executive, “The Control of Major Accident Hazards Regulations 2015,” L111 (Third edition), 2015, https://www.hse.gov.uk/pubns/priced/l111.pdf.
[6] Olsen, JE, “The process hazard analysis: An essential next step,” Process Safety Progress, August 2022, https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/prs.12409.
[7] Bridges, WG. “Best Practices for PHA Revalidations,” Presented at: 14th Global Congress on Process Safety; Orlando, FL; April 2018. https://www.process-improvement-institute.com/_downloads/Best_Practices_for_PHA_Revalidation_-_FINAL.PDF.
[8] Ozog H. Continuous PHA Revalidation. ioMosaic. 2006. https://iomosaic.com/docs/default-source/papers/continuous-pha-revalidation-2006.pdf?sfvrsn=6fc4cdd4_16. Accessed February 10, 2022.
[9] Miles JB, Process safety management at what point a work site would no longer be considered a modification but a new facility, Standard Interpretation, U.S. DOL OSHA website, January 11, 1996, https://www.osha.gov/laws-regs/standardinterpretations/1996-01-11, Accessed February 1, 2022.
[10] Miles JB, Steps for updating and revalidating a Process Hazard Analysis (PHA), Standard Interpretation, U.S. DOL OSHA website, June 22, 1998, https://www.osha.gov/laws-regs/standardinterpretations/1998-01-22, Accessed February 1, 2022.
[11] Bridges WG, Marshall M, “Necessity of Performing Hazard Evaluations (PHAs) on Non-normal Modes of Operation (Startup, Shutdown, & Online Maintenance),” Presented at: 12th Global Congress on Process Safety; Houston, TX; April 2016. https://www.process-improvement-institute.com/_downloads/Necessity_of_PHA_of_Non-Normal_Modes_of_Operation-R0.pdf, Accessed September 23, 2021.
[12] Medrano, Marco-Antonio, “A Methodological Approach to Perform Process Hazard Analysis (PHA) for Start-up of Chemical Processes,” Presented at: 19th Global Congress on Process Safety; March 15, 2023; Houston, TX.
About the Author
Jody E. Olsen
Principal engineer and Manager at JE Olsen Consulting LLC
Jody E. Olsen, P.E., is the principal engineer and manager at JE Olsen Consulting LLC. She provides process safety consulting services in process hazard analysis (PHA), management of change (MoC), pre-startup safety review (PSSR), root cause failure analysis, mechanical integrity, and other process safety management (PSM) elements. Prior to this role, she spent nearly three decades working in upstream oil and gas production for Mobil Oil, Aera Energy, and BP.