Better Data Promise Better Catalysts
For the first time in-situ monitoring of an electrochemically induced oxidation state change by X-ray photoelectron spectroscopy has been achieved, say researchers at the University of Nottingham, Nottingham, U.K. "As a result of this research, we can design more efficient catalysts, new probes, sensors, functionalized electrodes," explains Peter Licence, an associate professor in the School of Chemistry. The researchers succeeded in getting spectroscopic data for the electrochemical reduction of Fe3+ to Fe2+ in an ionic liquid mixture. Ionic liquids were necessary because they have negligible vapor pressures and so don't evaporate under the ultrahigh vacuum conditions employed.
Electrochemical Insights
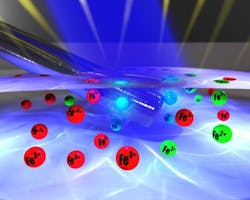
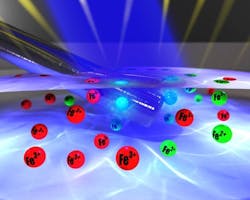
Figure 2. Artist's rendition of reduction of Fe3+ to Fe2+ at electrode shows x-rays (blue beam) irradiating surface from which photoelectrons escape. Source: University of Nottingham."It wasn't easy and we had phenomenal problems. We could do the electrochemistry in the vacuum and we could measure the spectra of ionic liquids — but to do both at the same time has been a real uphill struggle — but now we have cracked it," Details on the experiments appear in Chemical Communications.The next steps are to make the measurements easier to carry out and then to investigate other well-understood redox couples to further validate the system, he adds."We are using the in situ technique to study on a fundamental level the electronic structure of solution catalysts. We can use this data, in conjunction with laboratory data, to design better and more efficient catalyst. Essentially our technique closes a loop in a feedback mechanism — i.e., we can provide a direct feedback mechanism to electronic structure, allowing more efficient 'intelligent design' of new materials," he notes."The experiments carried out so far have proved the concept and shown that we can make the technique work," says Licence. "We are now looking at metal deposition/solution reactions and electron transfer vectors with an aim to study energy capture devices and electrochemically driven processes."