Catalyst Tuning Delivers Dramatic Results
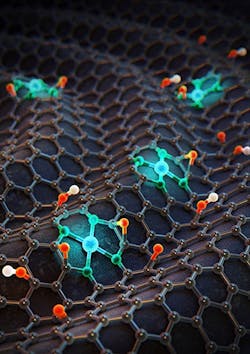
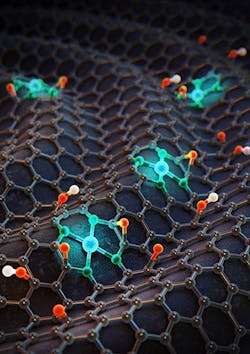
Figure 1. Three-dimensional image shows single cobalt atoms on nitrogen-doped graphene. Source: Institute for Basic Science (IBS), School of Chemical and Biological Engineering, Seoul National University.
A new electrocatalyst can produce eight times more hydrogen peroxide than conventional noble-metal-based electrocatalysts, report scientists in Korea. The tuning process they used to achieve that performance could profoundly impact industrial catalysis, they believe.The scientists, based at the Center for Nanoparticle Research, Institute for Basic Science (IBS), School of Chemical and Biological Engineering, Seoul National University, Seoul, created a new electrocatalyst comprising Co-N4 molecules incorporated into nitrogen-doped graphene (Figure 1). By tuning the electron density of the cobalt atoms with either electron-rich or electron-poor species such as oxygen or hydrogen atoms, they found the optimum structure for maximum hydrogen peroxide production.
The new catalyst’s raw materials cost as little as 1/2,000th that of the palladium, platinum and gold currently used. In addition, the catalyst boasts high stability — showing no activity loss over 110 hours of hydrogen peroxide production.
“Bear in mind that this activity comparison is not with the traditional anthraquinone process, which itself is a multi-step, energy intensive process that relies on expensive palladium catalysts. Electrochemical hydrogen peroxide production is an emerging field which can potentially substitute [for] this process,” notes IBS assistant professor Byoung Hoon Lee.
This would require two important developments: a cheap and scalable way to produce enough catalyst materials for industrial use; and a viable electrochemical cell system for large-scale production of high-purity hydrogen peroxide.
“We are working on the first issue and already have some results showing that the amount of catalyst needed can be prepared easily using the conventional synthetic processes used in the chemical industry. While we are not involved directly in electrochemical cell development, the field is growing rapidly and new solid electrolyte electrochemical cells show promise,” Lee adds. He believes both challenges will be overcome soon.
“The reason for low activity on industrially important heterogeneous catalysts is the lack of knowledge on the active site at the atomic level. With this work, we now have a deep understanding of how to identify active sites and design catalyst materials at the atomic level,” he explains.
In fact, the researchers already have applied the tuning concept to other industrially important catalysts, with results to be published in the near future.
“Several companies involved in the hydrogen peroxide production industry have contacted us, with some asking for meetings to discuss the possibilities of industrialization,” Lee concludes.