Mechanochemical Route Enables Faster, Greener Organosodium Production for Synthesis
A new mechanochemical synthesis method could help reduce the chemical sector’s reliance on lithium-based reagents by enabling cleaner, faster production of organosodium compounds. Lithium reagents play a central role in organic synthesis but are costly to extract and regionally concentrated, while sodium is abundant and easier to source sustainably.
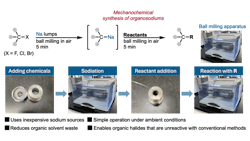
A team of researchers from WPI-ICReDD at Hokkaido University, Newcastle University and the University of Birmingham demonstrated that organosodium reagents can be produced by ball-milling organic halides with sodium metal using only a small amount of hexanes. According to the study, the process forms organosodium in about five minutes and avoids highly toxic reagents and moisture-sensitive conditions used in conventional methods.
The solid-state approach enables synthesis without inert atmospheres, and the team reported successful reactions with a range of substrates under the same mechanochemical conditions. The method also converted poorly soluble halides and fluorides that typically resist solution-based reactions, expanding potential applications in organic synthesis.
The research, published in Nature Synthesis, provides a pathway toward more sustainable organometallic production. The team notes that the findings establish groundwork for future development of organosodium chemistry with potential relevance to polymer, pharmaceutical and general synthetic workflows.