Manufacturing continues to evolve and has progressed through four stages thus far. It started with the implementation of steam power to mechanization. The second stage comprised mass production and the introduction of the assembly line powered by electricity. The third stage added computers and automation into the mix and, in the fourth stage, cyber-physical systems emerged to enable the computerization of manufacturing. This stage currently is evolving before our eyes and commonly is referred to as Industry 4.0.
“Industrie 4.0,” coined by the German government in a national strategy to promote the computerization of manufacturing, exemplifies the fourth industrial revolution on the way to an internet of things, data and services. Decentralized intelligence helps innovate intelligent object networking and independent process management, with the interaction of the real and virtual worlds representing a crucial new aspect of the production process.
The principle concept embodies connecting machines and systems to create intelligent networks along the value chain that control each other — for example, machines that predict failures and trigger maintenance processes autonomously or self-organized logistics that react to changes in production. Industry 4.0 technologies include many of today’s buzz-words like big data, advanced analytics, virtual reality, the cloud, the internet of things (IoT) and machine-to-machine (M2M) communication. In the past decade, these technologies have swept across the globe, as manufacturers worldwide recognize the value of Industry 4.0.
It certainly sounds great: a vision of the future with efficient, self-automated manufacturing processes that monitor themselves so they never go wrong.
A Developing Problem
It’s difficult to find fault with this formidable vision but a problem is brewing in implementing the principles of this industrial revolution. The potential for Industry 4.0 is so vast that a lot of companies rushing to adopt the technology haven’t paused to first figure out what root goals they are trying to achieve and problems they are striving to solve.
If these two issues aren’t addressed before embarking on the path to Industry 4.0, the route to follow is unclear; indeed, many companies are losing their way. The key technologies required for digital transformation cause radical changes in the business processes of any company. Those changes require discussion and understanding before they are undertaken.
For example, key stakeholders in the business must consider the company’s culture and how to help employees (at all levels) deal with change. This involves overcoming a general reluctance to change that’s typical of the human makeup as well as training employees to ensure they are qualified to use the new technologies. In addition, corporate leadership must address staff concerns about continued employment after digitalization is achieved by providing reassurances as appropriate and deciding if people can be redeployed in the new system (as often is the case).
Adopting Industry 4.0 involves a commitment and adequate resource allocation by the information technology (IT) department to ensure necessary connections are made and maintained, and to avoid any IT snags that could cause expensive production outages. Modern information and communication technologies like cyber-physical systems, big data analytics and cloud computing will foster early detection of defects and production failures, thus enabling their prevention and thereby providing productivity, quality and agility benefits that have significant competitive value. However, as more databases move onto — or connect with — the cloud, security issues commonly are cited as a barrier to fully embracing the new technology. It’s undeniably important to adequately protect all databases.
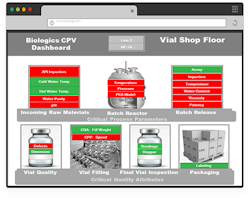
Figure 1. Dashboard provides a single red/green view of the entire manufacturing process.
Some software technologies necessitate the moving or duplicating of historian data or other data sources. This creates its own problems, both in terms of external data security and internal validation of the data. If a validated database is duplicated, does it need to be re-validated? These additional drains on IT and other resources demand consideration and discussion; sometimes an alternative approach is available that avoids these issues but still allows the company to move forward with digitization.
New technology must be reliable and stable for critical processes and operations and must meet any regulations applicable to the particular industry sector.
Ideally, a company can justify the economic benefits of this considerable investment and estimate an expected return on investment (ROI) for each stage and investments prioritized accordingly. In addition, it should ensure that all changes are based on strategic plans to place the company at an advantage or, at the very least, preserve current favorable market position.
Where To Begin
Like any journey, the path to Industry 4.0 should start with a map. From both strategic and technological perspectives, a company adopting these technologies must visualize every step on the route towards a digital enterprise. Success in the digital transformation process largely depends on a firm’s ability to prepare that plan in the most accurate way.
Dow is a great example of a major chemical company on its journey to Industry 4.0. Crucially, that journey started with an extensive effort to take better advantage of its data (see: “Data Initiative Improves Insights”) as well as embark on a review of processes and unmet needs. Dow looked at how best to maximize existing strengths, expertise and technology in moving forward to the next stage of its evolution.
For example, one plant suffered repeated upsets that resulted in unscheduled downtime. The site’s management realized that learning how to listen to signals the plant was sending to operators and responding better to those signals was key to addressing the problem. A team identified parameters of importance. This wasn’t an easy task. The amount of data was substantial — staff was spending eight hours per week gathering, analyzing and visualizing the data. However, these data were scattered in different databases, making it difficult to understand what was important. Analytics helped the team realize what the data meant and which parameters were crucial. The software provides operators with a simple green/red dashboard so they can quickly and easily see which parameters require attention and respond before product quality suffers or a shutdown results. Moreover, the analytics cut the time for gathering and analyzing the data to zero, freeing the production staff to focus on other things. The ROI was rapid.
In summary, in looking for a solution to issues that were impacting the bottom line through lost manufacturing time and the need for reprocessing, Dow set a goal to achieve a better data-driven understanding of parameters changing during the manufacturing process and the real-time effects of those changes — realizing such insights would lead to more-consistent more-efficient production. The success of this project reflects careful planning regarding the implementation and applicability of the new technology, setting the company on its path to Industry 4.0 in a useful and productive way.
Continuous Process Verification
The adoption of continuous process verification (CPV) by the pharmaceutical industry presents another ideal application for Industry 4.0 technology (see: “Control Systems: Take the Smart Road to CPV”). To meet regulatory guidance from the U. S. Food & Drug Administration and the European Medicines Agency, a pharmaceutical plant must continuously monitor hundreds — sometimes thousands — of variables to verify they remain within the parameters established and validated for its process. Industry 4.0 technology provides four key ways in which digitization can support CPV:
1. Analytics. Statistical process control techniques develop the data collection plan and statistical methods and procedures used in measuring and evaluating process stability and capability.
2. Risk-based real-time approach. This verifies a process produces material that meets all critical quality attributes and control strategy requirements.
3. In-line, on-line or at-line controls. These monitor process performance and product quality.
4. Quality attributes. Checking of feeds, in-process materials and finished products confirms they meet specifications.
Other benefits of digitization that apply especially to the pharmaceutical industry are the continual assurance of process control and the ability of data analytics to quickly detect any deviations from expected parameter limits. Such automatic monitoring and control gives a company continuous data for validation against regulatory guidelines, helping it comply with the rigorous associated requirements.
Five Key Steps
Our world is becoming increasingly digitized. This can be a good thing — improving efficiency, enhancing quality and helping a company meet ever-increasing data-related regulatory requirements. However, before embarking on the journey to Industry 4.0, a manufacturer should pause to consider the objectives of the exercise and set clear goals that will guide it on the way to successfully implementing — and sustaining — novel technologies. There’s no shortage of technologies. The crucial decision is choosing the one that’s going to provide the greatest positive impact on your company in the area that you most need it.
With this in mind, the five stepping stones to success on the path to digitization are:
1. Start with the problem. What challenge are you trying to address?
2. Pick an appropriate team. Which employees have the technical expertise to identify parameters of importance and how is your IT group going to help access key data sources?
3. Analyze the “people aspect” of the problem. Do you have the talent you need to achieve success?
4. Determine the best technologies. Besides being fit for purpose, do they maximize using existing technologies and data sources, provide a platform compatible with current equipment and offer scalability for the future?
5. Deploy effectively. Can you identify a pilot project for refining implementation and building confidence so you then can speed rollout to other sites?
By following these five steps, you can avoid common mistakes in implementing Industry 4.0, and set your company on the right path to a valuable and industrious new era of manufacturing.
PETER GUILFOYLE is vice president of marketing for Northwest Analytics, Portland, Ore. Email him at [email protected].