As the ethanol industry evolves, more plants are seeking ways to increase their product slate and margin. One option is manufacture of isobutanol and its derivatives including octane, jet fuel blendstock and other drop-in hydrocarbons. Commercial production of bio-based isobutanol, alongside ethanol, has taken place since 2014 at Gevo’s Luverne, Minn., facility (Figure 1). The side-by-side operation there shares various streams between the two production trains to minimize capital and operating cost. The facility currently is being upgraded to enable production of 1.5 million gal/y of isobutanol and 15 million gal/y of ethanol. We have restarted isobutanol production with an expected output of 750,000 to 1 million gallons of isobutanol in 2016. Once the process is fully optimized at this scale, isobutanol should yield EBITDA profit margins of approximately $0.50 to $1.00 per gallon, providing a compelling return on capital to the producer.
Figure 1. Luverne plant produces both isobutanol and ethanol.
Isobutanol is a colorless sweet-smelling liquid found naturally in fruits as well as in commercial ethanol fermentations and distilled spirits like whiskey. In contrast to ethanol, isobutanol has low water solubility and properties more like a hydrocarbon due to its chemical structure.
Isobutanol is commercially attractive both in traditional markets, such as solvents, coatings and chemical intermediates, and emerging ones such as fuel blends. Bio-based isobutanol has unique fuel properties and qualifies under the U.S. Renewable Fuel Standard for a Renewable Identification Number (RIN) — and thus should enjoy burgeoning demand in the fuels market. For example, due to isobutanol’s low water solubility, high octane and low vapor pressure, the marine market has strongly embraced isobutanol/gasoline blends. Indeed, the National Marine Manufacturers Association officially has endorsed Gevo’s bio-based isobutanol as a drop-in fuel for marine and recreational boat engines.
[javascriptSnippet ]
Isobutanol also can be chemically converted, using conventional unit operations, into isobutylene, which is a building block for isooctane, jet fuel, plastics, rubber, lubricants and other hydrocarbons. Switching to such products made from renewable raw materials is becoming increasingly important to companies looking to reduce their dependence on petroleum and improve the sustainability of the chemical business system. Moreover, as true drop-in replacements, the materials avoid the need for changes in assets and business models to adapt to a new molecule.
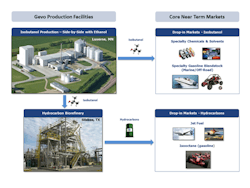
At South Hampton Resources, a company with a site in Silsbee, Texas, Gevo converts isobutanol to isobutylene, which then is used to make isooctane, isooctene and jet fuel (isoparaffinic kerosene) at the plant (Figure 2). Gevo has been producing and selling alcohol-to-jet fuel (ATJ) derived from isobutanol since 2011 when the fuel was used in certification trials, including test flights with the U.S. Air Force, Army and Navy. Gevo is working toward commercializing this ATJ production process. Isooctane made at this site has been sold into specialty fuels applications, including supplying Total with isooctane used in its Formula One racing fuels.
Isobutanol Production
Gevo’s process for making isobutanol from renewable feedstocks (Figure 3) relies on two proprietary developments: the yeast that produces isobutanol and a product recovery technology that continuously removes the isobutanol as it’s formed. Our yeast, which has been in development and optimization for over five years using the latest biotechnology tools, achieves commercially attractive yields and rates. Moreover, it is safe for use in animal feed, which is a key byproduct and source of revenue.
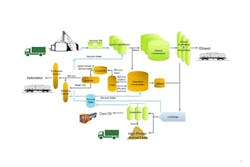
Figure 3. Several operations are shared by isobutanol and ethanol processes, cutting capital cost.
Feedstock for the process is biomass containing any fermentable sugars. At an existing dry-mill corn ethanol plant, the same corn mash stream coming from the front end of the process that feeds the ethanol fermentation is used for isobutanol fermentation. This stream goes to large non-sterile fermentation tanks (approximately 1 million liter at Luverne) where Gevo’s proprietary yeast converts the sugars to isobutanol. As with any alcohol fermentation, isobutanol has a stress effect on yeast as the fermentation proceeds and its concentration increases. Compared to ethanol, butanol is much more stressful on the fermentation organism. This typically would result in lower fermentation rate or reduced batch size. We avoid this problem by continuously removing the product during fermentation using Gevo Integrated Fermentation Technology (GIFT).
Even though isobutanol’s boiling point is 8°C higher than that of water, GIFT can remove isobutanol continuously during fermentation to maintain its concentration in the fermenter at target levels to optimize process rates and cost. This is accomplished by taking advantage of the azeotropic properties of isobutanol/water. The fermentation broth circulates through the GIFT where low-pressure evaporation occurs: the isobutanol flashes off the broth, resulting in a vapor concentration nearly 20 times greater than that in the fermenter. When the vapor is condensed, phase separation occurs because the isobutanol now is above its solubility limit in water — creating a relatively pure isobutanol-rich phase and a water-rich phase. The isobutanol-rich phase passes to a purification step. The water-rich phase goes to a stripping distillation step where residual isobutanol is recovered; the water from the stripper is returned to the fermenter. All the biomass remains in the fermenter-GIFT system, thereby greatly simplifying purification of the isobutanol.
In a typical ethanol plant, the water recycle and animal-feed operations can be shared with the ethanol process, thereby minimizing the capital needed for adding isobutanol capability. The spent fermentation broth from isobutanol production together with spent broth from ethanol production go through one set of decanters already in use in the ethanol process. The solids, which contain all the nutrients originally in the corn plus substantial additional protein created during fermentation, can be processed through an existing animal-feed dryer. Meanwhile, the liquids can go through a shared evaporation process, to yield a high-protein syrup, which is added to the animal feed, and an overhead stream of water for recycle in the plant.
The high-protein animal feed product provides an important source of revenue. For each 3,000 metric tons or 1 million gallons of isobutanol produced, the process yields nearly 4,000 metric tons of high-protein animal feed. That’s 20% more protein than comes into the process with corn feedstock. All of the nutrients in the corn end up in the high-protein animal feed, along with the additional protein created during fermentation.
Figure 2. Minnesota plant produces isobutanol and ethanol while Texas facility converts isobutanol into a variety of hydrocarbons.
The feedstock is No. 2 yellow dent corn, which represents about 99% of corn grown in the U.S. This corn currently goes for animal feed and industrial applications; none is used for direct human consumption. Many growers are employing more-advanced means of farming that can lead to a higher yields and lower inputs than conventional practices. A University of Minnesota study that assessed farm practices for a variety of growers near Gevo’s Luverne production facility concluded the corn grown by those farmers averaged 50% of the carbon footprint, in terms of gram CO2 equivalents/kg grain, of the “U.S. average,” as reported by the U.S. Dept. of Energy’s Argonne National Laboratory’s Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation Model. This is due to the high yields (188 bushels/acre in 2015) and relatively low inputs. Farmers that had the lowest carbon footprint used no-till farming and manure instead of other nutrients.
The isobutanol production process offers a number of key features:
• Continuous removal of product enables long fermentation times and large batches.
• Product recovery technology can be applied to C3-C6 alcohols.
• Any fermentable sugars in their unpurified form can be used, thereby avoiding capital and operating costs associated with sugar purification.
• Conventional dry-milling/starch feedstock as well as normal process conditions, enzymes and unit operations suffice.
• The high-protein coproduct is safe for animals and provides a significant revenue stream. Moreover, in a dry-mill operation, this process yields approximately 20% more protein than that in the feedstock.
• Yeast fermentation is robust — with no risk of phage infection, which is common in industrial bacterial fermentations.
The dry-mill-based production process in operation in Luverne now is being adapted to sugar mills for use around the world in a collaboration between Gevo and Praj Industries (India). Also, a consortium called the Northwest Advanced Renewables Alliance, which is funded by the U.S. Department of Agriculture, is adapting the process for use with woody feedstocks.
Gevo already has announced initial licensing partnerships with Praj Industries, Porta Hnos (Argentina), IGPC Ethanol (Canada) and Highlands Envirofuels (U.S.).
While Gevo will continue to produce isobutanol at its Luverne facility, we foresee licensing of our technology as the key driver of growth in isobutanol production. We expect this to occur via two key avenues:
1. Side-by-side. This approach allows an alcohol plant owner to add isobutanol production capacity to an existing site while leveraging infrastructure and operational efficiencies. These side-by-side plants would produce isobutanol, ethanol and animal feed, which should provide additional revenue and margin opportunities to the plant owner.
2. Retrofit. Alternatively, some ethanol plant owners might choose to switch all their fermentation capacity to isobutanol and cease production of ethanol.
Gevo’s ability to convert sugars from multiple renewable feedstocks makes isobutanol a global opportunity.
CHRIS RYAN is president and chief operating officer of Gevo Inc., Engelwood, Colo. E-mail him at [email protected].