Researchers at the U.S. Department of Energy’s Oak Ridge National Laboratory (ORNL), Tenn., are involved in two projects that could revolutionize our understanding of the catalysts and catalytic processes involved in biomass processing.
[pullquote]
The first involves zeolite catalysts that transform alcohols such as ethanol into higher-grade hydrocarbons. Scientists have been experimenting with the catalysts for decades, but ORNL researchers recently found the underlying reaction in zeolite-based conversions unfolds in a different manner than previously thought.
“For 40 years, everyone thought that these reactions must go first from ethanol to ethylene, and then from there it forms longer chains. We were able to show that it’s not how this occurs,” says ORNL’s Brian Davison, co-author of a study published in Nature Scientific Reports.
Figure 1. Chaitanya Narula led analysis of an ORNL biofuel-to-hydrocarbon conversion technology to explain the underlying process. Source: ORNL.
The researchers’ analysis found that this energy-consuming intermediary step isn’t necessary for conversion. Instead, an energy-producing “hydrocarbon pool” mechanism allows the zeolite catalysts to directly produce longer hydrocarbon chains from the original alcohols.
“It challenges a long-held, but incorrect, assumption,” notes ORNL co-author Chaitanya Narula. “It has been assumed that you must go from ethanol to ethylene, which is endothermic and requires energy. We showed this step doesn’t occur, and that the overall reaction is slightly exothermic.”
ORNL researchers tracked the molecular transition in labeling experiments with deuterium to confirm the hydrocarbon pool mechanism. They used a versatile heterobimetallic catalyst that completely converts ethanol to hydrocarbons at 250–450°C and atmospheric pressure without added hydrogen. The catalyst exhibits superior performance compared to monometallic catalysts as determined by low C2 yield and high durability.
“Our method of direct conversion of ethanol offers a pathway to produce suitable hydrocarbon blend-stock that may be blended at a refinery to yield fuels such as gasoline, diesel and jet fuel or commodity chemicals,” Narula explains.
The ORNL-developed catalyst and conversion process were licensed in 2014 to start-up company Vertimass, Irvine, Calif. Vertimass and ORNL are working to scale the technology to the commercial level.
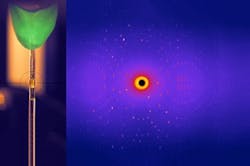
Figure 2. A glycosidase crystal (left) with a typical diffraction image resulting from neutron scattering. Source: TUM.
A second ORNL project is helping scientists understand the reaction mechanisms used by glycosidase enzymes.
When producing synthetics, intermediate chemical products or biofuels from biomass, hemicellulose, a large polysaccharide abundant in plant cell walls, must first be broken down to monomeric sugars. In contrast to the uncatalyzed reaction in neutral solution, enzymes such as glycosidases are able to speed up the decomposition reaction by up to 18 orders of magnitude.
The biomass is typically pre-treated in a very alkaline environment. However, natural enzymes develop their maximum activity in mildly acidic environments. An important research goal is to alter these enzymes to allow them to function effectively in a basic environment.
However, this demands intimate knowledge of how the enzymes function, in particular, the precise position of all hydrogen atoms in their action centers before, during and after the chemical reaction.
Using neutron sources based at the ORNL, the Los Alamos National Laboratory, New Mexico; and the Technical University of Munich (TUM), Germany, the scientists believe their results provide the key to improving large-scale technical processing of biomass.
“Unlike X-ray structure analysis, neutron diffraction is the method of choice when it comes to pinpointing the position of hydrogen atoms in the active center of enzymes,” says TUM biologist Andreas Ostermann.
The scientists analyzed the structure of the glycosidase using neutron scattering on enzyme crystals. They investigated the structure at various pH levels and in a complex with a ligand molecule. The researchers used insight gained from the neutron scattering experiments as a starting point for molecular dynamics simulations.
During their work they discovered that the decisive step depends on the orientation of one specific amino acid side chain, the free carboxyl group of a glutamic acid. When it turns down and away from the substrate, it can take over a proton from a water molecule. When the carboxyl group turns upward, the acidity is strengthened and the proton is transferred to the substrate.
The lead author of a report about the work, Andrey Kovalevsky, is very pleased with the success of the experiments: “No one has ever observed hydrogen atoms in a glycoside hydrolase enzyme, and until now we did not know how the catalytic glutamic acid residue is protonated.” Using this knowledge, work on altering the enzyme in a way that allows efficient biomass decomposition even in high pH environments can begin, he adds.