|
THIS MONTH'S PUZZLER |
| Our solvent recovery system uses a batch evaporator to concentrate the mother liquor from a crystallization process. The evaporator is a falling-film unit with a recirculation pump and a shell-and-tube heat exchanger. The concentrated solution (3% solids) is sent to an incinerator. At the end of a batch, the recirculation pump is used to transfer the concentrated solution to the incinerator feed tank. When the process originally was started up, the batch evaporation was run every 3 to 4 days, whenever enough mother liquor had accumulated. The process worked very well. Recently, due to increased production, the solvent recovery runs almost every day and the heat exchanger plugs up before the batch is finished. However, if the operators catch the fouling in time and let the solids settle, the run often can be extended. The solids content exceeds 10%, though, and the solids settle quickly, in under 15 minutes in a graduated cylinder. The incinerator can handle the higher solids content but only has limited spare capacity. Also, a boost in production is anticipated and the solvent recovery may need to be nearly continuous. We know that increasing the amount of concentrate to the incinerator is not desirable due to solvent losses and environmental regulations. Can you suggest a solution? |
Draw from a higher point
It appears that the problem is in pumping the slurry through the heat exchanger due to the high solids loading. A simple solution is to pull the solvent for recovery at a point high enough in the vessel to maintain low solids. Once the solids level rose enough, you could then pump from your current location (presumably the bottom) to the incinerator feed.
The puzzler did not specify the shape of vessel used to hold solvent awaiting recovery. If you are starting from scratch, consider a lamella separator with a conical bottom for pumping out the sludge or, if the solids are sticky, then an oversized conical tank with subsurface feed (below the takeout point for pumping to the evaporator).
Barry Bershad, senior process engineer
Clariant, Coventry, R.I.
Install an automatic filter
As solids seem to limit the capacity of the evaporator, why not install
some way of removing them? An automatic filter is one device that could work here if the solids are not sticky. The filter would discharge a concentrated stream to a collection system whenever the pressure differential across it reached a given set point. In this way, the evaporator could be operated continuously, resulting in greater throughput for this system. Admittedly this solution would require new equipment to be installed.
Jim Darby, process design engineer
LANXESS Inc., Sarnia, Ont.
Consider four options
First of all, Im not sure of the reasoning behind using a falling film evaporator to concentrate a mother liquor solution from a crystallizer. With the mother liquor being saturated, any increase in concentration by evaporation will result in crystal (solids) formation.
1. Why not send the mother liquor back to the crystallizer rather than the falling film evaporator?
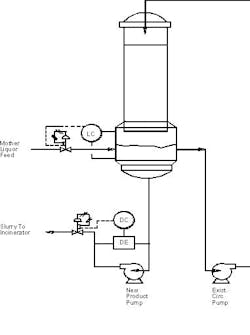
2. If the mother liquor cannot be sent back to the crystallizer and must be processed by the evaporator, then go to continuous operation and add a product pump with density control loop and modify the suction piping for the recirculation pump per Figure 1. With the rapid solids settling rate, the new product pump will discharge the slurry to the incinerator, and the recirculation pump will handle clarified solution.
3. It is possible that fouling in the heat exchanger is a result of plugging of the distribution plate (if a drilled perforated plate is used). If so, I recommend replacement with a shower-spray-nozzle distributor.
4. With an increase in production requirements, I recommend addition of a submerged-tube forced circulation finisher to operate in parallel in terms of vapor flow and series in terms of liquid flow. The solids concentration in the falling film would be reduced and the product concentration solution would be delivered by the forced circulation effect.
Joseph T. Badyrka, president
Evaporation Technical Services, Inc., Birmingham, Ala.
Try backflushing
Would it be possible to install a backflush system in your evaporator loop? It would work like this: A relatively clean solvent stream would be pumped backwards through the evaporator for about a minute every few hours (as needed). If this backflush idea worked satisfactorily, it could be automated a bit. It could be activated by a pressure switch (or something that detects plugging) or it could be put on an automatic timer.
Mark Otterson, project manager
Noveon, Inc., Avon Lake, Ohio
Go to a continuous crystallizer
Your solvent recovery/batch evaporation description doesnt state the relative value of those streams or whether the incinerator is dedicated to this stream only. However, incineration is always a net expense. Instead, remove the precipitated solids via continuous filtration from the recirculated reboiler stream, before the heat exchanger. You can then landfill or perhaps even sell the contaminant-containing solids at a discount. The bonus is that you can recover much more of your solvent this way. Even if your solvent or solids have appreciable fuel value, they are almost always more valuable as process materials.
However, the real way to solve your problem is to step back and look at your overall operation. It sounds like you may have reached the production scale where you should use continuous crystallization, with a bleed stream of the crystallizer liquor to control impurity concentration. You may be able to increase product purity, since the mother liquor will now contain lower impurity levels (if the impurity solubility curve is flatter than the product solubility curve). Now, you can run your falling-film evaporator continuously, thereby avoiding storage of spent mother liquor and recovered solvent. You might even be able to blend some of the recovered waste solids back into a portion of your product, if you sell different product purity grades. Add in carbon adsorption/recycle of your vented solvent vapor and you have eliminated all need for incineration and for air-quality operating permits.
Lisa Miller, principal process engineer
Icosa Company, Inc., Greensburg, Pa.
Opt for a press
Use an inline press to concentrate the solids to 50% and landfill the solids. Circulate the liquor in the evaporator through the press continually. If heat is a problem and the membrane wont hold up, use a vortex concentrator to move the solids to an off-process tank and then, after the material cools, run it though the press.
John E. Adams, facility engineer
GE Appliances, Louisville, Ky.
Recycle concentrated solution
Having a solvent recovery system on a crystallizer is an ideal situation to recover the solvent and the solids from the mother liquor. Based on the problem description, it would be correct to assume that the solids in the solvent are same as the solids that are being crystallized. If this were the case, my first choice would be to recycle the concentrated-solid-containing solution to the crystallizer. The temperature and flow rate of the concentrate would have to be controlled such that it does not disturb the crystallization process. If the crystallization process is run continuously, recycle the concentrate as crystallizer feed. You will gain two benefits, i.e., total recovery of the solvent and the solids, an ideal solution.
Girish Malhotra, P.E., president
EPCOT International, Pepper Pike, Ohio
|
JANUARYS PUZZLER |
|
Why did a thermowell in a recirculation pipeline near the discharge of a pump indicate a change in temperature sooner than a thermowell inserted via a top nozzle about half way down into a well mixed vessel? |