Prevent Formation of Ignitable Mixtures
Many chemical makers use flammable and combustible liquids and, thus, face a serious risk of a fire or explosion during the handling, processing and storage of these liquids. If containment is lost, then, depending upon the quantity released, local ventilation or draft conditions, ambient temperature and volatility of the liquid, ignition of the vapors and subsequent fire or explosion could result in severe injuries or loss of life and damage to the facility and the environment.
So, plant personnel must understand the hazards that flammable liquids present, such as:
• Materials that are easily ignited by several types of ignition sources, including electrostatic discharges. Less than 1 mJ of spark energy — what a perceptible doorknob shock provides — can ignite most vapor/air mixtures.
• Liquid vapors that can readily mix with air to form explosive mixtures and can easily travel along the floor from one location to another or get raised to higher levels due to air currents.
• Liquids that burn readily and give off more heat than fires involving ordinary materials like paper or wood; the resulting high temperatures often make it very difficult to extinguish fires involving these flammable liquids. Also, the products of combustion generally are noxious.
• Vapors from flammable liquids that mix with surrounding air and burn with explosive force when confined.
• Flammable liquid fires that can spread quickly depending upon the extent of containment and the slope of the floor or ground.
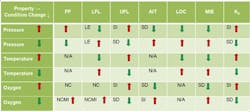
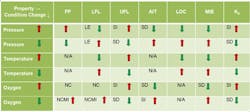
Table 1. Various flammability properties of liquids and vapors change as pressure, temperature or oxygen level increases or decreases.
LE = little effect; NC = no change, NCMI = no change until nose of curve then minimal increases; SD = significant decrease; SI = significant increase; and NA = not applicable]
Three elements are required for a fire to occur: fuel; an oxidizer, such as the oxygen in air; and an ignition source. When all three elements are present in one location and at the same time, then the risk of fire exists. Controlling or eliminating one or more of the elements greatly minimizes the risk of a fire. In other words, a fire won’t happen if flammable vapor isn’t present within its ignitable range; the oxidant concentration can’t support combustion; or the ignition source lacks sufficient energy.
Two additional elements — confinement and mixing of the vapor with air — are needed to create an explosion hazard.
Flammability Properties
To assess the risk of fire or explosion — and to identify the safety precautions needed to minimize the hazards — you must determine the flammability characteristics of the liquids and vapors. Safety-critical flammability properties include:
• flash point (FP) or lower temperature limit (LTL);
• lower and upper flammable limits (LFL/UFL);
• auto-ignition temperature (AIT);
• limiting oxidant concentration (LOC);
• minimum ignition energy (MIE); and
• maximum explosion pressure (Pmax), rate of pressure rise (dP/dt)max and deflagration index (Kg).
Processing conditions, e.g., temperature, pressure and oxidant concentration, affect flammability characteristics such as FP, LFL, UFL, AIT, LOC, MIE, Pmax, and Kg (Table 1).
Very limited data on the flammability properties of chemicals or chemical mixtures at low and elevated temperature and pressure are available from public sources. So, most situations require experimentally determining flammability properties under representative process conditions.
Reducing Fire and Explosion Hazards
To safeguard process equipment against the risk of a fire and explosion, process designers should adopt a “layers of protection” approach.
The first layer should focus on preventing the formation of flammable mixtures (this is the most desirable basis of safety) by:
• controlling the fuel concentration (LFL and FP), or
• reducing the oxidant concentration to below the limiting oxidant concentration (LOC).
The second layer should control ignition sources (desirable, but challenging and outside the scope of this discussion).
The third should mitigate fire/explosion consequences (an “add-on” to support the first two layers) through explosion containment, venting, suppression or isolation as well as equipment spacing or barricading. (Fire/explosion mitigation also is outside the scope of this article.)
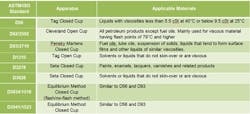
Table 2. The nature of the material determines the appropriate test method to use.
Let’s look at how to control the fuel concentration and reduce the oxidant concentration.
Control Of Fuel Concentration
If practical, the liquid should be maintained a temperature less than its FP, which is the lowest temperature, corrected to the standard atmospheric pressure of 760 mm Hg (101.3 Kpa), at which sufficient vapor exists above a liquid surface to form an ignitable mixture with an oxidant (usually the oxygen in air). Keeping the temperature below the liquid’s FP will ensure that not enough of its vapor exists above the liquid surface to create a fire or explosion hazard.
FP increases with increasing pressure and decreases with decreasing pressure. It also depends upon the type of oxidant (air versus chlorine, for instance) and the oxidant concentration.
You can measure FP using either open-cup or closed-cup methods. Closed-cup FP data usually are preferred to open-cup data because the closed cup prevents the loss of vapors to the atmosphere before the application of the ignition source; hence, the results are more conservative. Note that the configuration of the test apparatus, sample size, ignition source, sample-heating rate, sample homogeneity, drafts and operator bias can impact the measurement. Table 2 lists the basic standard methods for measuring FP. Figure 1 shows a Pensky Martens closed-cup apparatus.
Figure 1. This closed-cup device can determine flash point temperature for a variety of materials.
If you must measure FP at a pressure other than atmospheric or in an oxidant gas other than air, using one of the standard methods may not be feasible. Instead, a special/custom test design may need to be developed.
Operating below the liquid’s FP may pose process challenges, including:
• Lowering the process temperature to under the FP could affect the final product.
• Substituting with a lower-FP material may impact the final product or require extensive research and development.
• For processes in which the order that flammable and combustible liquids are added to the vessel isn’t important, adding the higher FP liquids first may not be effective in raising the FP of the mixture if the bulk of the mixture contains the lower-FP material or the process temperature is at or above the highest-FP material.
• If operating below the FP of the liquid requires a process temperature lower than ambient, you must consider the design, installation and operating costs of the necessary heat exchanger, along with the costs for its inspection, cleaning and maintenance.
Table 3. A variety of methods can reduce oxidant concentration to below the LOC.
Reduction In Oxidant Concentration
The LOC can be defined as the minimum concentration of an oxidant required to propagate a flame through a homogenous gas/vapor mixture. Lowering the oxidant concentration to below the LOC can prevent fires and explosions, regardless of the fuel concentration.
According to “NFPA 69: Standard on Explosion Prevention Systems,” where the oxidant concentration is continuously monitored, a safety factor of 2.0 vol-% generally is recommended unless the LOC is less than 5.0 vol-%, in which case the equipment shall be operated at no more than 60% of the LOC. Where the oxidant concentration is not continuously monitored, the oxidant concentration within the vessel should not exceed 60% of the LOC, or 40% if the LOC is below 5 vol-%.
To reduce the oxidant concentration to a pre-determined safe level, you can use several purging methods: vacuum purging; pressure purging; combined pressure-vacuum purging; sweep-through purging; and siphon purging. Table 3 describes these methods.
Each method has advantages and disadvantages:
• Pressure purging is faster because the pressure differentials are greater; however, it uses more inert gas than vacuum purging.
• Vacuum purging uses less inert gas because the oxidant concentration primarily is reduced by vacuum.
• Combined vacuum-pressure process uses less inert gas compared to pressure purging, especially if the initial cycle is a vacuum cycle.
• Pressure, vacuum or vacuum-pressure purging can only be used for vessels or equipment that have appropriate ratings for the positive pressure or vacuum conditions.
• In the sweep-through process, the purge gas passes through the vessel continuously. This simplifies operation but consumes more gas, which could make the method relatively expensive. Also, this technique may not suit all types of processes, especially if the material handled is toxic, if a high-vapor-pressure material is present or if the inert gas is not properly mixed within the headspace above the liquid surface.
• Siphon purging can help minimize the gas expenses associated with sweep-through purging.
Reducing the oxygen concentration below the LOC normally can’t be used to prevent flash fire or explosion in occupied spaces, such as rooms or buildings. However, with forewarning and actuation-delay systems, flooding otherwise-occupied rooms with purge gas may be a viable option.
Making The Right Choice
Controlling fuel concentration to below the FP and reducing oxidant concentration to under the LOC is normally preferable from a safety standpoint to controlling ignition sources and mitigating fire/explosion consequences.
When you must choose between the two options, consider controlling fuel concentration first. This is because reducing oxidant concentration generally involves an inert gas (nitrogen, carbon dioxide, halons, etc.), and so poses an inherent asphyxiation hazard if containment of the inert gas is lost. Controlling fuel concentration also is better when you can make process changes to operate below the liquid’s FP or if you can use a material with a FP higher than the process temperature.
However, in cases when the process design, materials handled or operating conditions may make preventing formation of a flammable mixture within equipment impractical, you must rely upon controlling ignition sources or mitigating the fire/explosion consequences as the primary “basis of safety.” I strongly recommend that you conduct a detailed hazard assessment on the process to determine the layers of protection needed.
As highlighted throughout this article, our approach to the assessment and control of fire and explosion hazards involves utilization of process-safety-related data/information/models and knowledge/experience. We routinely use many types of information, such as flammability, toxicity and reactivity of chemical substances, in managing process safety. This information must come from data that have been validly obtained and are appropriate to the situation, bearing in mind that operating conditions, contaminants, equipment characteristics and processing sequences can impact the validity of data.
ANAND KENCHENPUR is the flammability group manager at Chilworth Technology, Inc., Princeton, N.J. E-mail him at [email protected].