The majority of the new biopharmaceuticals and those in development today are produced in complex mammalian cell cultures — typically involving tens of biochemical species and hundreds of biochemical reactions. These biologics such as therapeutic proteins and monoclonal antibodies now account for more than 70% of the top-selling treatment drugs.
Success in efficiently performing the complex and interdependent metabolic reactions critically depends upon the precise makeup of the cell culture medium and the nutrient feed regime, which controls the medium composition throughout the duration of the drug production process.
The biopharmaceutical industry long has recognized that nutrient feed control is crucial to process development lead-times and to manufacturing quality, yield and cost. Real-time measurement of physical parameters such as pH and dissolved oxygen is insufficient to achieve dynamic feed control. Instead, companies still depend upon predetermined nutrient regimes or periodic extractive sampling and offline analysis, resulting in suboptimal conditions for cell culture, process delays and risk of infection.
Figure 1. Probe provides real-time data that analyzer uses to generate a control parameter for nutrient addition.
The majority of biopharmaceutical processes use a fed-batch nutrient-feeding regime to maintain cell culture activity. Current fed-batch processes rely on predetermined feeding protocols based on nutrient requirement estimates or infrequent sampling and offline measurement of the culture media to determine the concentration of key components. Both these techniques can lead to nonoptimal feeding, with depletion of nutrient and large swings in nutrient concentration, risking the health of the organism and its ability to produce consistent product quality at a high concentration. While attempts have been made to automate these feeding control protocols, the inherent nonoptimal limitations remain. So, developing timely cost-effective manufacturing processes for new biopharmaceuticals requires a radically new approach to feeding control.
A Paradigm Shift
Real-time monitoring of the metabolic activity of the process enables a fundamentally different approach that both automates the process and optimizes nutrient feeding. Metabolic activity is a function of nutrient concentration. For the majority of cell types used in upstream bioprocesses, an increase in feedstock concentration results in an increase in activity until a maximum metabolic rate is reached (the so-called “Monod relationship”). It therefore is possible to use a decrease in metabolic activity as an indicator for reduced nutrient concentration and a trigger for timely feed additions.
This automated closed-loop feeding control (AFC) model differs significantly from the current fed-batch model in two respects. Firstly, the process is monitored continuously without the need for extractive sampling. This “tightens” the control loop and eliminates the risk of process infection caused by sampling. Secondly, using the metabolic activity of the cells to control the feed additions means the cells themselves directly govern the process. This ensures the cells are maintained in a steady more-productive state than is the case with larger, more infrequent nutrient swings. Reducing the levels of cell “stress” increases the efficiency of the process resulting in higher yield. “Happy cells” deliver more product.
Successful Application
As part of an ongoing collaboration with the Biopharma Research Group at the GSK Medicine Research Centre, Stevenage, U.K., over a twelve-month period Stratophase applied its Ranger AFC system to a Chinese hamster ovary (CHO) cell culture. GSK was instrumental in the development of the feeding control methodology and its supporting applications software, which runs on the Ranger Manager. As a result of this collaborative effort, Stratophase has been able to reduce AFC setup time to a matter of weeks, which is now benefitting other companies.
The system is commercially available and currently is supported by direct personnel in Europe and the United States. Stratophase’s manufacturing facility in the United Kingdom designs and assembles all system components.
The Ranger system (Figure 1) consists of a Ranger Probe, which is mounted within a bioreactor, and a Ranger Manager, which takes the real-time data, referred to as the process trend index (PTI), from the probe. The Ranger Manager applies sophisticated mathematical methods to analyze the PTI to generate a control parameter reflecting the metabolic activity, referred to as the metabolic rate index (MRI). This MRI control signal triggers a feed pump, which delivers, nutrient-rich media additions to the bioreactor. The closed-loop control is fully automated without the need for manual intervention.
Other sensors measure specific parameters such as temperature, pH and dissolved oxygen that often are used to control the physical state of the reactor but no other sensor provides a real-time view of the process metabolic activity.
Although the Ranger system does not require sampling to operate, samples were taken daily for offline analysis during the GSK trial. Comparing the real-time Ranger data and the offline assay data gave insight into the AFC technique and the resultant conditions under which cell cultures are maintained.
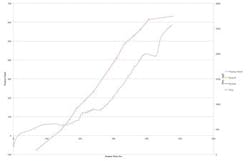
Figure 2. Process trend index generated by automated feed system nicely matches results of cumulative feed profile determined by offline sampling.
Figure 2 shows the PTI plotted against the cumulative feed profile generated by AFC. While they are different parameters, the PTI and the offline cumulative feed profile correlate with each other. Observable changes in the PTI match the short-term upticks in feedstock. The cell culture initially was run as a batch, with AFC set to start controlling at 96 hours. The first feed was triggered at 148 hours; regular feeding began at 196 hours. The PTI profile shows upticks associated with feed addition and the characteristic consumption phases between feed pulses.
A number of discrete stages can be observed as the process progresses:
1. 196–236 hours show feeding with consumption phases commonly associated with the PTI being dominated by the nutrient consumption.
2. 263–390 hours show feeding with consumption phases commonly associated with the PTI being dominated by product evolution.
3. 396–420 hours show a lack of feeding. This coincided with the feedstock running out, making feed addition impossible despite the Ranger triggering the pump.
4. 420 hours onwards show a characteristic and significant upturn in PTI, which is due to a decrease in cell viability.
Figure 3 plots the PTI against product titer. As in Figure 2, the PTI correlates well with data from offline assay. The PTI, while not a direct measure of product titer, clearly reflects the dominant effect of the product within the media. Product evolves at a consistent rate throughout the process. From 263–390 hours, when the consumption phases of the AFC cycle are product dominant, a clear correlation exists between the increasing PTI and the product accumulated within the media. The decrease in product release rate at a point beyond 405 hours reflects the nutrient limitation caused by the feedstock running out and a fall in cell viability. The rapid increase in PTI at the end of the process is symptomatic of cell death, with an associated reduction in rate of production of titer.
A Better Way
The cell culture outlined in this article and the implementation of the AFC technique demonstrate the value of the Ranger system for controlling an on-demand feeding strategy in real-time that meets the culture’s requirements throughout the duration of the process. The use of a single feed, consisting of nutrient-rich media, ensured the Ranger system successfully responded to and arrested any decrease in metabolic activity associated with reduced concentrations of the critical components within the media. In this specific case, the AFC supported both cell growth and production phases by automatically adjusting to the requirements of the cell without the need for user intervention.
Other customer trials also demonstrate the value of the Ranger system for optimizing feed rates and minimizing nutrient concentration variations.
Figure 3. The process trend index also correlates well with product titer.
AFC has led to consistently high degrees of cell health (happy cells), higher product titers and uniform product quality throughout the process — all without the need for user intervention. It enables a decrease in process development time and increase in product output that can play a major role in providing cost-effective treatments.
BILL CAMPBELL is CEO of Stratophase Ltd., Romsey, U.K. E-mail him at [email protected].