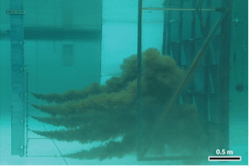Separations are the largest source of U.S. industrial emissions and consume 10%-15% of the world’s energy [1]. Porous polymer membranes are low-cost, energy-efficient materials for separations and are used extensively in water treatment and purification. Membrane performance is determined in large part by the surface properties, especially along the pore walls.
Commercial membranes are typically hydrophobic, making them prone to fouling – a failure mode where the pores become clogged with organic/bio materials. For water treatment and industrial separation facility operators, fouling is a vexing problem and necessitates costly replacement or cleaning of that increases operating expenses.
Most existing solutions to address membrane fouling entail replacing existing polymers with new materials, requiring significant changes to the manufacturing process. An alternative strategy is to coat the inner surfaces of existing polymer membranes with fouling-resistant materials. However, most coating methods produce non-uniform coatings, especially within the micron-scale pores, leading to blockage and reduced permeance.
To address the problems of typical coating methods, scientists at Argonne National Laboratory have adapted a technique commonly found in semiconductor microelectronics manufacturing called atomic layer deposition (ALD). Using ALD, we coat polymer nanofiltration and ultrafiltration membranes with extremely uniform, ultrathin coatings (~1 nm) of metal-oxide ceramic materials, such as alumina and silica. These metal oxides are naturally hydrophilic but bond weakly with organic contaminants in water.
Fouling Resistance With Atomic Layer Deposition
These properties allow the polymer membranes to resist fouling, so they maintain a high permeance and are much easier to rinse compared to uncoated polymers. Surprisingly, ceramic materials, such as alumina and silica, are remarkably pliable when applied as an ultrathin film, so the polymer membranes maintain their flexibility.
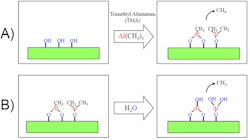
The ALD technique exposes the polymer membrane to a series of chemical vapor pulses. In the case of alumina (Al2O3), the first vapor would be trimethyl aluminum (TMA) and the next would be H2O. In simplified terms, the TMA deposits a monolayer of aluminum on the surface, and the water deposits the oxygen to make Al2O3. More precisely, the TMA reacts with functional groups on the polymer surface, such as hydroxyl (OH), species to deposit a layer of aluminum terminated with methyl (CH3) groups.
An essential aspect of ALD is that the TMA is unreactive toward the methyl groups, so the film stops growing once a saturated monolayer forms. During the ALD process (Figure 2), TMA molecules diffuse into the membrane pores to reach sites deep within the membrane. The subsequent H2O exposure chemically reacts with the methyl groups to form methane and deposit oxygen terminated with hydroxyl (OH) groups. This TMA/H2O sequence deposits a monolayer of Al2O3 and regenerates the OH-terminated surface, so we can repeat the cycles until we achieve the desired film thickness. Approximately 10 TMA/H2O cycles are necessary to grow 1 nm of Al2O3, which is typically all that’s required to convert the hydrophobic polymer surface to hydrophilic Al2O3 and impart the fouling resistance.
Stronger Surface Coating With Sequential Infiltration Synthesis
Argonne has also developed a related technology for functionalizing polymers known as sequential infiltration synthesis (SIS). This technology is similar to ALD in that it uses cyclic chemical vapor exposures, but in this case, the chemicals diffuse below the polymer surface (i.e., through the free volume) where they chemically react with the polymer functional groups. The effect is that the ceramic material grows inside of the polymer rather than strictly on the surface. SIS technology can be used to make organic-inorganic hybrid materials with unique properties, but in the context of water treatment and separations, SIS creates strong, covalent bonds between the polymer and the ceramic so that the resulting surface coating is mechanically and chemically robust.
Argonne used SIS and chemical grafting of an oleophilic monolayer on polyurethane foam to create Oleo Sponge. Oleo Sponge is a reusable sorbent that rapidly and efficiently separates oil from water. Argonne qualified Oleo Sponge at the Ohmsett National Oil Spill Response Research and Renewable Energy Test Facility in New Jersey by separating crude oil from seawater under winter conditions (Figure 3).
For even more challenging fouling environments, Argonne has developed catalytically active ALD coatings to form reactive oxygen species at the pore walls, which degrades and detaches any foulants that adhere. Through ALD, Argonne also has developed gradient coatings along the pore lengths, producing so-called “Janus membranes” with different properties on their two sides. These membranes can exhibit transport properties that are impossible to achieve using traditional membranes, such as the unidirectional transport of ions.
Operations using ALD and SIS coatings could apply them as a final step in an existing manufacturing process for polymer membranes to add desirable surface properties, such as fouling resistance, that are missing from the polymer.
Although ALD and SIS on polymer membranes are at the research and development stage, clear pathways exist to large-scale manufacturing and commercialization. For instance, ALD is typically performed using a heated vacuum system in a batch mode, which would be too costly for membrane manufacturing. However, research has shown promise for room-temperature and atmospheric ALD for many materials. Also, roll-to-roll ALD coating systems that can be adapted for polymer membranes are commercially available.
Large-scale, efficient manufacturing of ALD and SIS coatings on polymer membranes would benefit a broad range of sectors, including desalination, agriculture, municipal water treatment, the oil and gas industry and chemical manufacturing. Replacing energy intensive processes such as distillation with membrane-based separation would use 90% less energy and reduce carbon emissions by a factor of 10 [1].
References
1) D. S. Sholl and R. P. Lively, “Seven chemical separations to change the world”, Nature, 532, 435–437 (2016).