A process that encapsulates particles in a sieve-like film to block unwanted reactants could improve the reactivity and selectivity of oxide catalysts say researchers from Northwestern University, Evanston, Ill., and Agronne National Laboratory, Lemont, Ill. They foresee it resulting in greener, more efficient conversion of biomass and glucose into fuels and other fine chemicals.
"The ability to conduct these reactions in a selective way opens doors to new applications in green chemistry and sustainability," says Justin Notestein, assistant professor of chemical and biological engineering at Northwestern's McCormick School of Engineering. "Unlike current processes, which may require enzymes or precious metals, our method relies only on harmless, inert oxides."
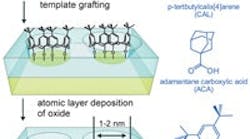
The researchers focused on photocatalytic oxidations to test their method. They deposited a template on a core particle of titanium dioxide and applied a nanometer-thick film of aluminum oxide around but not on the template using atomic layer deposition. Removing the template then left the film with tiny holes, or "nanocavities," less than two nanometers in diameter (see Figure 1).
NANOCAVITIES
Figure 1. A sieve-like surface allows access only to molecules small enough to penetrate the <2nm-diamater supermicroporous cavities. Source: Christian Canlas, Northwestern University.The coating acts like a sieve, allowing only the smaller reactants in a mixture to slip through the holes and react with the titanium oxide, while larger reactants were blocked. The result was much higher selectivity (up to 9:1) toward the less hindered reactants. More details can be found in a recent article in Nature Chemistry. Research is ongoing and the next step is to expand the application, "in particular, using more traditional redox-active and acidic oxides, and develop alternate synthesis methods, including those that do not use atomic layer deposition," says Notestein. "We expect that within two years we will have developed a number of targeted versions of this first, proof-of-concept catalyst system," he adds.The process was conducted at room temperature and required only a low-power light source. In fact, temperature limits are likely those of the phase transformations of the oxides composing the catalyst, says Notestein. "Although not discussed in the published manuscript, these materials are well behaved after a typical calcination step at 500°C." He also notes that other researchers at Northwestern and Argonne have developed a related technique that helps stabilize supported metal catalysts against coking and high-temperature sintering.Notestein says that because the sieving layer is only as thick as a single molecule, they haven't experienced any clogging in the traditional sense, but "like any other catalyst, it can be poisoned by strong chemisorption." "Optimizing catalyst formulations for specific applications will remain the primary challenge for some time. We expect that some of the more reactive catalyst surfaces will have challenges in template selection and film growth."Notestein believes the chemical compatibility of the sieving layer with the reactants and solvent is likely the biggest limitation, but also the greatest opportunity. "If an oxide used as the parent catalyst or the sieving layer is incompatible with the solvent, for example, an additional layer can be added to passivate most of the surface," he explains.The team expects the materials to serve as drop-in replacements for other oxide catalysts and to offer comparable service lives for the particular application. "We hope that by improving selectivity we will decrease coking, improving catalyst lifetime before regeneration is needed," says Notestein.To further control and improve selectivity, the team is looking into template molecules that are smaller or have larger aspect ratios. "Like a zeolite, we hypothesize that closer approach to molecular dimensions will improve reactant and product selectivity," says Notestein.The researchers have no immediate plans for pilot-scale testing.