THIS MONTH'S PUZZLER
We're having trouble with the vertical thermosiphon reboiler of the debutanizer column of our fluid catalytic cracker (FCC). The heating medium is the fractionation column bottoms, which arrive at the reboiler shell at a nominal 297°F, following cooling from 682°F in a steam-generating kettle reboiler at the discharge of the fractionation bottoms pump. During startup and shutdown, the discharge from the bottoms pump briefly spikes to 700–715°F. Last year, we abandoned in-place a corroded debutanizer kettle reboiler and have had problems ever since with the thermosiphon. We pull its tube bundle every 8–12 weeks to clean the fouling inside the tubes; now, we're having trouble pulling the bundle. The fractionation column bottoms pump, which was upgraded with a larger impeller and motor at the same time the kettle reboiler was abandoned, is seeing a drop in head, running loud and tripping vibration alarms. What's going on here? What can we do to improve the cycle time of the FCC?
CHECK THREE ASPECTS
Here are a few things to consider and investigate:
1. In reference to the exchanger you abandoned in-place for the new thermosiphon reboiler, was a hydraulic analysis conducted to validate if the location of the new thermosiphon exchanger and its associated piping and fittings support natural circulation of the tube-side fluid? There may be some hydraulic impacts that could cause an unstable thermosiphon operation. In addition, make sure the liquid level in the column sump is relatively constant to avoid cycling the thermosiphon due to a hydraulic imbalance caused by density differences and percent vaporization.
2. In reference to the fouling you are experiencing, what is the temperature difference between the two fluids in the thermosiphon? Normally, debutanizer column bottoms boil at 250–260°F at ~ 66 psig. What pressure does the debutanizer column operate at? The lower the pressure in the column bottoms, the lower the boiling point will be, of course. As a result, based on the heating medium you are using at 297°F, the mean temperature difference will be higher and could cause a rapid boiling through the tubes. Gums and heavy-type residue could polymerize from rapid boiling, which would prematurely foul the exchanger. If you're experiencing high spikes in the fractionation column bottoms temperature during transient operations, startups/shutdowns, then the temperature differences between the two fluids in the thermosiphon could result in rapid boiling, potentially facilitating polymerization in the tubes.
3. What are the constituents in the fractionation column bottoms used as the heating medium for the vertical thermosiphon? If there are heavies that, when cooled, can plate out in the shell, this could cause high shell-side fouling, making the tube bundle difficult to remove. Consider a heating medium that's more consistent in temperature and is clean when cooled, i.e., low pressure (LP) steam. LP steam isn't normally subjected to spikes and could reduce fouling due to reduced temperature differences compared to the debutanizer bottoms boiling point temperature. This may require a new thermosiphon design due to condensing steam, because the heat transfer condensing coefficient for the steam will be higher than that of the fractionation bottoms stream if there's no phase change. If you're fouling on the shell side, this will impact your pump performance as well.
Eric Roy, principal engineer
Westlake Chemical Corp., Sulfur, La.
BLAME HIGH TEMPERATURE
Clearly, the fouling is occurring on both sides of the thermosiphon reboiler. For the reboiler shell, the source of the fouling is the FCC reactor beds; for the tube-side, the fouling probably is the polymerization of the C5+ olefins. As the fouling inside the tubes continues, the heat transfer coefficients drop, temperature profiles become less uniform, and polymers are converted to coke and varnish that are difficult to remove. The reboiler shell fouling is a witch's brew of catalyst fines (carryover), coke particles and polymerized olefins. Take samples from tubes and shell — and clean both next time.
What are the root causes of this poor cycle time? Perhaps the catalyst selection or the operation of the reactors are culprits; spikes resulting from poor temperature control in the fractionation column may be occurring more often because of poor tuning or even soot coating the thermocouples — these coatings could mean that all temperature readings here are lower than actual. Then, there's the persnickety nature of thermosiphons.
Thermosiphons are limited to a turndown of about 2:1 — unlike a valve tray, which can handle a quarter of its nominal capacity and still produce good product. A sudden change in column bottom level, composition or pressure, as is likely to occur during a shutdown or startup, can cause flow oscillations and even mechanical vibration, non-uniform temperature gradients and poor heat transfer coefficients. For example, if the bottom accumulates compounds of low volatility, like coke, unstable flow will occur in the thermosiphon. Sudden changes in a tray tower such as a rise in pressure can result in a drop in vapor velocity in the tower; vapor velocity is inversely proportional to tower pressure, which should also increase the flow from the thermosiphon because of residual heat. One oscillation can feed on another.
Let's not rule out design errors completely because this is a new exchanger. It could be that the suction and discharge lines are undersized. It's important to remember that thermosiphons rely on two-phase flow at the discharge not the inlet, so check the size of both lines. Partially closing the suction line can temporarily curtail oscillation and could be a good check that oscillation has occurred. Another design issue could be the locations of the inlet and outlet.
Maybe, the kettle reboiler is a better choice if it can be replaced or refitted; certainly, it would be better to have two reboilers if fouling is an issue.
Another problem is the fractionation bottoms pump. From the description of lost head and vibration, it seems that some kind of erosion/corrosion is at work. Take this pump out of service immediately for non-destructive and destructive testing. A lined replacement should help; however, a closed impeller may be a better choice because it provides better protection to the leading edge than an open impeller. A good design practice is to maintain a velocity in the 9–12-ft/s range in all exchanger tubes to prevent fouling; this unfortunately requires hardening the material of construction to survive the erosion. Erosion is proportional to the cube of velocity.
Dirk Willard, senior process engineer
Superior Engineering, Hammond, Ind.
AUGUST'S PUZZLER
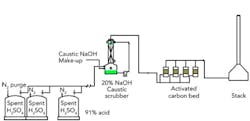
We're trying to settle a dispute between our on-site contractor and the company designing a vent scrubber for our alkylation spent acid tanks (see Figure 1). The design basis is a flow of 3,500 pounds per hour (pph) of a stream containing H2S (about 60 pph), N2, water, O2, 1,3 butadiene, 1,4 pentadiene, acetaldehyde, formaldehyde and other components from the tanks to a caustic scrubber and activated carbon bed (ACB) before going to a stack. This composition seems inaccurate but it's what we have. The gas molecular weight is 29.7 going into the scrubber and 29.1 exiting it. The initial pressure is only 3.2 in. water column (WC); the designer claims the pressure drop through the scrubber will be only 1 in. WC and that the drop through the ACB will be 0.5 in. WC. The designer proposes using a 3-in. schedule-40 carbon steel vent to convey vapor from three spent H2SO4 tanks, a distance of 175 ft.; the same 3-in. pipe will carry fumes 250 ft to the scrubber and then to the ACB 80 ft away; the stack is 120-ft high and 24-in. in diameter. The design doesn't include a blower or fan because of the fire risk, which also is why the firm chose steel rather than fiberglass pipe. It also lacks a flame arrestor before the scrubber; the designer says API-2210 doesn't require one because the existing conservation vent will provide sufficient protection. However, the contractor contends the conservation vent must be re-specified for the proposed design changes, the 3-in. pipe won't allow 3,500 pph, carbon steel will corrode and is, in fact, unnecessary, i.e., fiberglass will do because the vent isolates the vent system from flash back (agreeing with API-2210). The contractor also argues the pressure drops in the scrubber and ACB are too low. What do you think? Does the design need changing? If so, how?
Figure 1. Designer and contractor disagree about whether this is an acceptable design.