Simulated Moving Bed Chromatography Offers Real Attractions
Simulated Moving Bed (SMB) chromatography is a continuous purification technique that has higher throughput and requires less solvent than regular batch chromatography. Even for difficult separations, it can achieve high yield and high purity at a reasonable production rate.
The process was developed in the 1960s for the purification of sugars from molasses. Since then it has won a role in the pharmaceutical industry for the purification of enantiomers from racemic mixtures [1]. In recent years, interest in the technology by both industry and academia has grown. As a result, SMB applications have expanded beyond the sugar and racemic separations to more complex separations.
Today, a few active pharmaceutical ingredients (APIs) are produced using SMB. However, some obstacles prevent the broader application of the technology. This article will present these obstacles and how to overcome them to perform successful separations.
Process principle
SMB is a chromatographic technique based on a flow of liquid (mobile phase) moving countercurrent to a constant flow of solid (stationary phase). Countercurrent flow enhances the potential for the separation and, hence, makes the process more efficient. It also allows a continuous flow of feed material to be separated, which improves the throughput of the equipment compared to traditional batch chromatography.
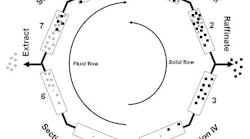
Providing a constant flow of solid is impractical in a production process. Therefore, the solid instead is packed into high pressure columns. These columns are arranged in a ring formation made up of four sections with one or more columns per section (see Figure 1). Two inlet streams (feed and eluent) and two outlets streams (extract and raffinate) are directed in alternating order to and from the column ring. Because the columns cannot be moved, the inlet and outlet position is switched at regular time intervals in the direction of the liquid flow, thus simulating countercurrent movement of columns.
The flow rates in Sections II and III are important because this is where the separation occurs. Sections I and IV handle “cleaning.” Mobile phase exiting Section IV is directly recycled to section I. The solid is regenerated there by desorbing the more retained compound with a high flow rate so the complete column can be “moved” into section IV.
The SMB process can be modeled with the appropriate mass-balance equations for each column and the adsorption isotherms for each compound. Experimental data are used to verify and adjust the model. Simulations are then conducted to design the equipment to satisfy the required production.
How the technique compares
A single enantiomer can be manufactured by either an asymmetric route, such as biocatalysis or asymmetric synthesis, or by purification of a racemic mixture via crystallization, chemical resolution, chiral membranes or chromatography.
The first option requires the discovery of the right enzyme or catalyst. Enantiomeric ratios are not always good and asymmetric techniques require long and expensive process development. However, the theoretical yield is 100% because only the desired enantiomer is prepared.
Racemic purification is usually a simpler route because the synthesis of the racemic is less complex. Crystallization processes require enrichment of the desired enantiomer in the mixture and often result in low yields. Chemical resolution involves three steps: making a salt, performing the separation and then recovering the product from the salt. Even if the yields are good, each step introduces a cost to the final API. Chiral membranes are still in their early stages and are limited to certain solvents and small molecules. In contrast, method development for chromatography is very fast (a couple of weeks) and scale up is straightforward, making it attractive. The theoretical yield for these methods is 50% because both enantiomers are present; however, if racemization is possible the yield can be increased, thereby improving the process economics. It is not uncommon to find that SMB processes are more economical at commercial scale than any other option.
One of the great advantages of chromatography compared to other processes is the capacity to scale up linearly. A process demonstrated at pilot scale can be reproduced very quickly at production scale without sacrificing purity and production rate.
SMB showstoppers
Chromatographic processes are still considered as the last resort, to be selected only when everything else fails. Several hurdles remain to be overcome before this technology becomes widely used in a production environment.
Image. Chromatography is often considered expensive and not applicable at large scale. This misconception is based on chemists’ experience with low pressure columns, where separations can indeed be very slow and consume a lot of solvent. Modern batch chromatography and SMB are more efficient techniques. Large diameter columns (up to 1,000 mm) operating at 30-40 bars are in use in the industry for both batch and SMB. Several metric tons of API (enantiomers) are produced every year using SMB chromatography at a lower cost than traditional separation techniques.
Regulatory issues. The pharmaceutical industry is heavily regulated. All processes must be perfectly defined and validated. As a consequence, batch processes usually are favored because tracking of material is easier. SMB is a continuous process and, hence, raises many questions from quality groups. However, several processes for the manufacturing of APIs using SMB have been approved by the FDA and other regulatory agencies.
Reliability. A SMB unit is a complex assemblage of valves, pumps, pipes and columns. This complexity causes concerns about downtime, especially considering the continuous nature of the process. A good preventive-maintenance program can minimize downtime. Aerojet Fine Chemicals has achieved an on-stream factor of 95% over 15 months on its commercial-scale SMB unit, demonstrating the reliability of the technology.
Process robustness. Careful selection of various parameters is crucial. The pumps’ accuracy is essential for the robustness of the separation. A small change in the flow rate can have an impact on the final product purity and must be evaluated. The same type of evaluation should be conducted for variation of the eluent or the feed composition, the column performance [5] and the separation temperature. Assessment of these parameters should pinpoint a range of operating conditions that will ensure that product quality is maintained.
Packing material. The mechanical and chemical stability of the packing material during long campaigns is always an issue. If the material deteriorates over time then replacement will be required. Fortunately, the use of dynamic axial compression allows for long column lifetime. It is not uncommon, however, to see the performance degrade slowly over time, necessitating repacking of the columns with new material.
Sometimes trace-level impurities such as residual solvents or high-molecular-weight compounds are present in the feed material. These impurities can either slowly erode the packing material or be strongly absorbed onto it. Either results in a reduction of the separation performance. Eventually the separation will not be possible and regeneration or replacement of the packing will be necessary. The use of a pre-column can help increase column lifetime and, hence, reduce the cost associated with the packing. The packing replacement must be carefully evaluated and included in the cost of the final API.
Attractive applications
SMB now should be considered for a number of applications:
Enantiomer purification. SMB is ideal for the separation of enantiomers. APIs purified by chromatography have been validated and approved by the FDA. Because SMB can provide high purity and high recovery in a very short time, it is an excellent way to reduce time to market.
The cost of the packing material used for chiral separations is often a hurdle in the early development phase. When the production reaches larger scale, the stationary phase can serve for several years and, hence, can be amortized over a longer period. Thus, its relative cost per kg of product becomes small.
Other applications. Any binary-like separation can be a good candidate for SMB.
Separation of diastereoismers sometimes can be achieved via SMB. This often can be economically attractive because cheap packing with high loading capacity can be used.
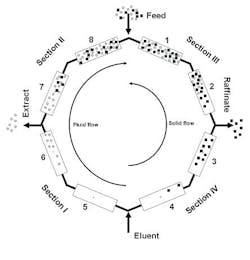
In the recent years, applications to remove a small amount of a single impurity have been developed. Bioxel Pharma has recently published information on the use of a new high-containment SMB unit at Aerojet Fine Chemicals (Figure 2) for the purification of Paclitaxel (Taxol) from its closely related impurities [6].
SMB also can handle more complex separation such as purification of proteins. However, some modifications to the standard unit design are necessary to allow for packing regeneration [7].
As SMB technology becomes more widespread, a larger variety of applications undoubtedly will be developed and publicized.
Real potential
The pharmaceutical industry is investigating SMB as a production tool, attracted by its fast method development and easy scale up. A few roadblocks still exist but they are fading away as more and more units find their way into both industry and universities. Contract manufacturing companies now use SMB units at different scale and major pharmaceutical companies are installing their own production units. As the technology evolves, such systems will become more of a routine unit operation rather than the “niche technology” of a few experts.
Dr. Kathleen Mihlbachler is research scientist in MS&T for Eli Lilly and Company, Indianapolis, Ind. E-mail her at [email protected].
Dr. Olivier Dapremont is director, chromatographic separations, for Aerojet Fine Chemicals, Rancho Cordova, Calif. E-mail him at [email protected].
REFERENCES
1. Miller, L., C. Orihuela, R. Fronek, D. Honda and O. Dapremont, “Chromatographic resolution of the enantiomers of a pharmaceutical intermediate from the milligram to the kilogram scale,” J. of Chromatogr. A, 849, Issue 2, p. 309 (1999).
2. Seidel-Morgenstern, A., “Experimental determination of single solute and competitive adsorption isotherms,” J. of Chromatogr. A, 1,037, Issues 1-2, p. 255 (2004).
3. Ruthven, D.M. and C.B. Ching, “Counter-current and simulated moving bed adsorption separation processes,” Chem. Eng. Sci., 44, p. 1,011 (1989).
4. Storti, G., M. Mazotti, M. Morbidelli and S. Carra, “Robust design of binary countercurrent adsorption separation processes,” A.I.Ch.E. J., 39, p. 471 (1993).
5. Mihlbachler, K., A. Jupke, A. Seidel-Morgenstern, H. Schmidt-Traub and G. Guiochon, “Effect of the homogeneity of the column set on the performance of a simulated moving bed unit. Part II: experimental study,” J. of Chromatogr. A, 944, p. 3 (2002).
6. Metz, P. and O. Dapremont, “SMB takes on Paclitaxel,” Pharma. Mfg., 3, Issue 9, p. 27 (Nov/Dec 2004).
7. Xie, Y., S. Mun, J.-H. Kim and N.-H.L. Wang, “Standing wave design and experimental validation of a tandem simulated moving bed process for insulin purification,” Biotech. Prog., 18, p. 1,332 (2002).
8. Mihlbachler, K., “Enantioseparation via SMB chromatography: a study of Tröger’s base unique adsorption behavior and the influence of heterogeneity of the column set on the performance of the SMB process,” Logos-Verlag, Berlin (2002).