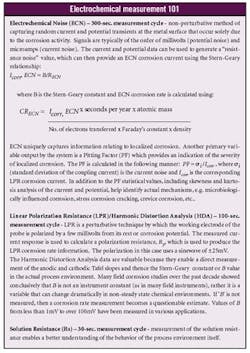
BASF Benefits from Online Corrosion Monitoring
BASF Corp. is using SmartCET, an online, real-time monitoring technology developed by InterCorr International Inc., Houston, to troubleshoot and prevent corrosion problems at its Freeport, Texas, site more effectively. The technique offers significant advantages compared with conventional corrosion-monitoring methods.
Today, most plants use very low-tech, labor-intensive methods to gage corrosion, typically checking the condition and material loss of "coupons,"sample pieces of metal of a set size, weighing and measuring, then reweighing and remeasuring them to assess damage.
Figure 1: Electrodes, shown here, were inserted in pipelines to allow continuous monitoring.
And even this technique provides only a retrospective check rather than continuous monitoring capability, according to Dawn Eden, marketing manager at InterCorr. Offline measurement techniques using electrical or linear polarization resistance only provide data periodically, and require that users download, then process, data.
BASF installed SmartCET at an organic chemical processing unit at its Freeport site, a complex made up of 19 different plants producing such mainstays as acrylic acid, oxo alcohols and polycaprolactam. This specific unit, constructed mainly of carbon steel, 304L and 316L stainless, was experiencing significant corrosion problems after decades of debottlenecking and other process modifications.
Both general corrosion and localized pitting were problems, and even after corroded steel piping and equipment had been replaced with 304L, then 316L stainless steel, the problems continued, says Joseph Kintz, senior materials engineer for BASF. The results were unscheduled and expensive shutdowns and costly downtime.
"We weren't sure what was causing the corrosion,"Kintz said in a presentation at a recent conference at the National Assn. of Corrosion Engineers' (NACE's) central region in Houston. The exposure environment was a hot, volatile, mostly organic process stream containing approximately 1% water, caustic, and possibly entrained solids and gases. Caustic and high temperatures were obvious potential problems, but operators suspected other culprits, including catalyst, peroxide and entrained solids and gases. "There were many unknowns," he said.
Figure 2: A closeup of an 8-in. probe.
In August 2001, a cross-disciplinary team of process, project, operations, maintenance and materials specialists first met to analyze the plant's corrosion problems. After studying historical information, tracking changes that had been made to the chemical process, and examining actual pieces of corroded equipment, the team soon realized that more information would be needed to get to the bottom of the problem, given the complexity of the process. For one thing, the process is hot and highly flammable, posing safety issues. Furthermore, its economics wouldn't justify rebuilding the facility with more corrosion-resistant materials -- i.e., replacing carbon steel with stainless, or stainless with nickel-based alloys.
BASF's team then considered corrosion coupon testing -- briefly, Kintz said, but realized that it would take many years and "lots of money" to discover the causes via that approach. Monitoring corrosion online with electrical resistance probes wasn't the way, either. "When previously used, measurements typically didn't agree with experience,"Kintz said. "We needed to find out what it was about the process that was causing the corrosion, then see if the process could be modified to run under acceptable production rates with acceptable rates of corrosion,"Kintz said.
Figure 3: Shown here, an operator installs a 2-in. probe.
After convincing management of the need to take action, and studying the alternatives, the BASF team decided to install SmartCET at the facility. Installation was very quick -- a matter of days, according to InterCorr's Eden. There were some initial start-up problems with the system related to electrical noise carried by the plant's piping. These were eventually resolved and the system has worked well ever since.
Flange-style probes (Fig. 1) were customized for the facility to allow operators to monitor pipe surfaces at all times. The flange style was chosen for its durability and because it would allow the entire circumference of the pipe to be monitored at all times, an important consideration, since the pipe-wall corrosion was nonuniform.
The probes were installed in an 8-in.-diameter stainless steel pipeline where the most severe corrosion had been observed (Fig. 2). Probes made of carbon steel, 304L and 316L stainless steel were then inserted in the pipeline (Fig. 3), downstream of the caustic water addition and the injection point for another minor process stream. To meet safety requirements, the corrosion analyzers were placed in explosion-and weather -proof enclosures (Fig. 4).
Surprising corrosion sources
Data from the monitoring system were analyzed, and a correlation was found between the LPR corrosion rate of carbon steel and the pump-down schedule for a particular upstream vessel. The vessel was put on an automatic pump-down schedule so that it pumped its contents into a reactor approximately once per hour. The level of liquid in the reactor was automatically controlled so that, when it received increased flow from the vessel, it automatically increased its discharge flow rate. The reactor discharged a relatively small quantity of a mixed organic/aqueous stream into the primarily organic stream being monitored. Every time the vessel pumped down, the corrosiveness of the larger stream increased.
Several years ago, the facility's operators had reduced the concentration of a particular neutralizing chemical in the process. Now, they thought that this reduction might have been responsible for unusually high corrosion rates.
Using the system to monitor corrosion rates, a test was run to see weather increasing the concentration of the neutralizer would reduce corrosion rates. Quite the opposite happened. Increasing amounts of neutralizer dramatically increased corrosion rates rather than reducing them. This new information not only helped to reduce corrosion rates, but also provided chemical engineers at the plant with new insight into the chemistry of the process.
During the process, parts-per-billion concentrations of catalyst are added to enhance the chemistry of the process. After noticing a dramatic increase in corrosion rates, a plant technician pointed out that the increase occurred right after a new batch of catalyst was mixed. The procedure used to mix the catalyst resulted in small variations in catalyst concentration, and the online monitoring system allowed those variations to be tracked along with the corrosion rate.
The corrosion rate was shown to vary quite significantly with process and operational events. For example, the Pitting Factor and LPR corrosion rate show an inverse relationship when localized corrosion is occurring. Monitoring of general corrosion alone could not have brought these relationships to light. In addition, operators found that the LPR corrosion rate of carbon steel was directly connected with the amount of a key gaseous chemical used in the process, whose addition level inadvertently varied.
Another process stream monitored at the plant is primarily aqueous with normally low corrosion rate. Early in the testing program, short-term spikes to relatively high corrosion rates were sometimes observed. The corrosion rate spikes coincided with pumping of laboratory samples upstream of the corrosion monitoring probes. When plant operators changed their procedure and disposed of lab samples differently, the corrosion spikes ceased.
Future applications
Operators have taken baseline ultrasonic thickness measurements on various parts of the piping in the vicinity of the corrosion probes. After enough time has elapsed to show reasonably accurate wall-thickness losses, the data will be compared with the cumulative metal loss data to assess the accuracy of the real-time corrosion measurements.The company is considering the system for other plant applications, for instance, corrosion troubleshooting work, chemical treatment of open-circuit cooling tower water.
So far, BASF has found the system effective for monitoring corrosion behavior, Kintz says, and expects to be able to detect and resolve corrosion problems before significant equipment damage occurs rather than reacting once the damage is done.
Figure 4: The probes were housed in explosion-proof and weatherproof (show above) enclosures.
PIMS Integration
In January 2003, the system was integrated with the site's process information management system (PIMS), enabling plant operators and engineers to view real-time corrosion data alongside standard process variables (temperatures, pressures, flow rates, vessel liquid levels). During the early phases of corrosion testing, a list was made of process variables that could affect corrosion rates. Experiments were then run by adjusting one process variable at a time while monitoring the effect on corrosion rates and pitting factors.
This method soon revealed an intricate relationship between process variables and corrosion behavior. Many process changes influence corrosion, and combinations of process changes produce unexpectedly complex effects on corrosion. In order to address this complexity and maximize the efficiency of the testing program, the site began using the multi-variable testing (MVT) protocol. With this system, a set of process variables is randomly modified all at the same time. BASF has identified 11 key process variables, and will run 12 experiments with a specific process recipe to identify the variables that influence corrosion and synergies between them, Kintz says. This type of corrosion evaluation would not be possible without real-time data.