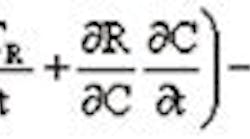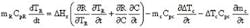How Can We Better Control an Exotherm? | Chemical Processing
Oct. 23, 2003
We have to hold tight temperature control on a highly exothermic suspension polymerization process in order to maximize reaction rates. We tried using PID on the catalyst flow, but temperature fluctuation was too high. We added PID control to the cooling water and can now control the temperature within +/-5 Degrees C, but now the batch time is too long. What can we do to achieve tighter control on this kind of process?,"From August Chemical ProcessingFocus on heat transfer
where subscript R refers to the reactor conditions and subscript c refers to the coolant conditions. It is critical to note that in polymerization reactions the rate of reaction, R, is typically a highly nonlinear function of both TR and the catalyst feed rate, C, while the cooling capacity is determined by the flow of coolant. In addition, the ability to cool the reactor is limited by the reactor surface area and the overall heat transfer coefficient.There are a number of options to improve reactor cooling.First, one may marginally improve the overall heat transfer coefficient by increasing agitation speed. Since the main purpose of the agitation is to maintain the suspension, a higher agitator speed should not hurt the process, although it might improve the mass transfer from the bulk liquid into the reaction sites and result in a higher reaction rate. Higher turbulence at the wall will enhance the heat transfer slightly, but I believe that this would not be sufficient to improve control and reduce batch time.Second, one can change to a cooling media with a higher heat capacity. This option will be capital intensive in that a new system is required.Third, by adding a pump-around loop to the reactor vessel one can add additional heat exchange surface by either jacketing the pump around or putting a heat exchanger in-line. This option might not be practical due to increased maintenance resulting from fouling the heat exchange area.Fourth, find a suspending fluid that will boil at the appropriate reaction temperature to take advantage of the latent heat effects. The vapors should be condensed and returned to the vessel. This will require an intensive development effort.Fifth, scale down the process. A smaller reaction vessel will have a larger surface area to volume ratio. This will have the effect of increasing the heat transfer capability of the reactor, and ultimately allow shorter batch times with better temperature control. In addition, two smaller reactors will probably provide more throughput than a single reactor of equivalent size. This option might be difficult to sell to management due to capital investment and the potential for higher operational cost brought on by increasing the number of operators.
Keith Dackson, Ph.D., Engineering Scientist
Henkel Technologies/Loctite Electronics, Olean, N.Y.We successfully scaled up a highly exothermic and "unusual" batch isomerization reaction from 1 liter in a calorimeter to a 30-gal. pilot-scale batch and from there to a 500-gal. full-scale reaction. We were able to abandon a $20-MM plant modification based on a continuous reactor and substituted a $5-MM (1980s $$) modification based on a couple of batch reactors. Learn From a scale up
I would proceed as follows:Run the reaction in a batch-heat-flow/heat-compensation calorimeter. This let's you run the reaction under conditions where the heat-transfer surface to reaction-volume ratio is very large. You can run the reaction in batch or semibatch mode; you can add the catalyst all at once, or add it slowly, i.e., you can explore heat evolution (and yields) as a function of time under various conditions. Since heat evolution is always some function of reaction rate, you're actually getting a real-time view of the reaction kinetics.Once you have these data, first determine how large a surface-to-volume ratio you need to prevent a runaway reaction. (Remember - batch reactions are always potential bombs, since during an upward temperature excursion, the heat transfer capability increases linearly with cooling media Add even more heat transfer area so that you can run at a relatively small Even if you only have a jacket, you must pump the cooling media around - so that it is essentially isothermal. The cooling loop acts as big flywheel. Cooling media temperature is adjusted by adding coolant to the pump-around loop and bleeding off an equivalent amount.Should the cooling media become too cold (e.g., during a process interruption), you need the capability of heating the pump around loop to get the reaction started again.Initially, when our ChEs tried to run the isomerization on a 10 gal. scale, they ended up with two runaways out a total of four reactions. And even for the successful ones, it was like balancing an egg on a pin.There was a real incentive to go batch,"it was much cheaper. I finally built my own heat-compensating calorimeter in one afternoon (a 1 liter resin kettle, submerged in a temperature-controlled bath, with an electric immersion heater in the reactor for temperature control). It was 1980, before commercial calorimeters were available. During the first run, we found that the reaction was not 1st order (as we had suspected). Here is what we observed: Initially, heat release was quite slow. Then, after about 30 min., the heat release started to pick up, but then diminished exponentially (as expected). It was that 30 min. phenomenon that caused the runaway reactions.There was obviously an "initiation" period, where an intermediate compound was formed, which then further reacted to complete the isomerization. We never determined the mechanism (it wasn't really important) because we now knew why we had the runaway reactions. We scaled up directly to a 30 gal. pilot-plant reactor, with a pump-around loop. We actually started out at a higher temperature, to get the reaction going, and as the heat release increased, we lowered the reaction temperature to maintain a constant heat evolution.On the second 30-gal. run, we only made a single temperature adjustment to the cooling loop,"and the reaction ran perfectly. When we then scaled up 500 gal. commercial batches (which required an external heat exchanger), we never had any problems with temperature control.
Gary Knapp, President GFK Consulting Ltd.,
San Clemente, Calif.Subject to limitations due to reactor volume or agitation requirements, use a high-heat-capacity inert such as tabular alumina spheres to dampen temperature swings. Tabular alumina spheres of 1/2 to 3/4-in. diameter would provide a significant heat sink, a high void fraction and would not significantly impede flow from the reactor at the end of a cycle.
Bill Norman, Technology Manager
Porocel Corp., [email protected]I think you meant solution polymerization. In the past, I have used an external cooler (heat exchanger) installed in the reactor circulation line. Depending on polymer properties and cleaning considerations, monomer injection can be on the inlet (preferred) or outlet of the cooler.It's the media's fault
Add external cooling
Monomer injection should be interlocked with high/low temperature on the reactor to prevent run-away exotherm and accumulation of unreacted monomer. Circulation pump and cooling should also be interlocked.
Robert Bryant II, Manufacturing Engineer
Valent USA, Walnut Creek, Calif.For a tight temperature control we often use a loop with a relative high-flow-capacity pump (about 5 to 10 times the vessel content per hour) and feed the loop with fresh cooling water over a PID-controlled three-way valve. This is a good and reliable solution in case the amount of heat to be removed gives a relatively small temperature rise in the cooling water. That's why this flow has to be as large as possible. The background of the story is that the heat buffer capacity of the cooling water is so large that the response time becomes also large. This gives the control loop the time to adjust the temperature before it runs away. I hope you can do something with this suggestion. However, the mixing action of the agitator is also a very important variable in this problem and in most cases the bottleneck.
Alan Ferraro, V.P. Engineering
Jongia Mixing/MPE Group USA, Pennsauken, N.J.The reader is right about PID control. You can get rapid response, but have too much overshooting and swings or you can control the overshooting and swings but have long process times and very sluggish response. Most exothermic reactions only allow a very narrow temperature band but require a rapid response to "catch" and control the reaction when it occurs. Allowing the exothermic reaction to get too hot or too cold can ruin the product batch. It is a significant issue with polymers.Lots of water flow buffers response time
Consider vacuum steam
We have a patented system that can control to +/- 1.8 Degrees F (1 Degrees C) and provide instant response to process fluctuations and temperature profiles. It is especially well-suited for exothermic reactions since it provides both heating and cooling capabilities. The heating system provides vacuum steam as low as 10 Degrees F. A pressure control valve uses proprietary control algorithms to allow rapid, measured response with minimal overshooting and swings. The cooling system uses an atomized water spray, which vaporizes under vacuum conditions to take advantage of the latent heat transfer at a much lower temperature (below 32 Degrees F if using brine solution). Product cooling times can be reduced by about 25%. Additionally, these systems provide energy and space savings when replacing hot water systems. By the way, did you know that vacuum steam has more BTU/lb. to transfer in latent heat? Just an added bonus.
Steve Kortheuer
TLV Corp., Charlotte N.C.Initially this sounds like the temperature setpoint (PID control) on the cooling water is too low. But I'm sure there is more to it than that. I suspect there is more than one process parameter that controls the reaction time.Vary the setpoint
Perhaps the process requires maintaining one temperature when in steady state but could be allowed to raise a given amount when the catalyst is added. If this is the case, the controller could switch to a higher setpoint when triggered that the catalyst is being added. The setpoint could go back to the initial value after a set time period. Some PID controllers can store more that one setpoint and switch them based on time or logic.For other situations, consider an over-ride controller. This consists of two PID controllers on one box that share an output (or set of outputs if both heating and cooling are controller). A simple rule set determines which PID controller had control of the output. Usually this is based on which controller output is calling for the lowest output, but this is configurable. Over-ride allows one output to be independently controlled by two parameters via two PID controllers.
Jim Overturf, VP of Marketing,
Eurotherm Inc., Leesburg, Va.The problem is that a tight temperature control is difficult to achieve by indirect cooling through a jacket of the vessel ," because of the high energy produced by the reaction, the heat to be removed is also high. The limitation to removing that heat will be mainly on the product side (not in the jacket).Improve the stirring
One simple way to improve the heat transfer is stirring. But in order to stir, the solution or suspension has to be diluted. Thus the concentration of active substance is lower and so too is conversion and reaction speed. If the suspension is particularly dense, the agitator will not move or, using a tougher, kneader type unit, the induced energy will be eventually even higher through mechanical dissipation.One appropriate method to overcome this problem is evaporative cooling. This means the operating pressure is adjusted so that one of the components in the reaction mixture (likely the solvent or suspension media) simply boils. You will need a closed design of the equipment and pressure control. The evaporated solvent can be recycled internally through a simple heat exchanger. You have to check if there is danger that one of the active substances of the reaction is stripped out and chose your solvent accordingly.
Daniel Witte, Senior Process & Sales Engineer,
List USA Inc., Charlotte, N.C.If a liquid is exposed to a pressure lower than its steam pressure, it starts evaporating and radiating heat through the developing vapors until the steam pressure is equal to the ambient pressure. In this process, the liquid cools down to the temperature corresponding to the prevailing pressure.Vacuum recirculation cooling
If the homogeneous mix of the product is exposed to underpressure, water will evaporate from the mix until the pressure of the water is equal to the interior pressure in the mixer. With the developing vapor, a heat stream is dissipated from the paste in the form of evaporative heat. At this point, the paste cools down to the temperature corresponding to the prevalent pressure according to the water vapor chart.With our vacuum cooling method, the cooling is generated in the product itself, independent from cooling surfaces (contact cooling) or from environmental conditions (evaporative cooling).A rotating mixing pan and rotary mixing tool keeps the product constantly moving, thus supporting the evaporation process.The temperature of the prepared product in the mixer is determined by the generated vacuum (underpressure), because pressure and temperature are related according to the water vapor chart. With a pre-selected defined pressure value, it is possible to automatically control the product temperature by means of the process controller.Occurrence of partial temperature differences in the product is impossible. The product quantity is entirely exposed to the vacuum, so that the water evaporates evenly throughout. The water vapor originated during the product cooling process is evacuated through a surface condenser, which consists of a bundle of thin-walled tubes with external circulation of cooling water. The developing condensate flows immediately back into the mixer, again being mixed homogeneously into the product. The processes of evaporation, condensation, and recirculation are occurring simultaneously during the cooling phase.
Nick Semitka
Eirich Machines, Gurnee, Ill.The problem does not lie in the method of control (PID in this case) or the tuning of the controllers. It is a heat transfer issue. Consider the enthalpy balance:DT, while heat generated increases exponentially with reaction temperature). If your reactor has insufficient jacket area, incorporate a pump-around loop to get sufficient heat exchange area.DT between cooling media and reactants. By doing this, the cooling media temperature essentially determines the reaction temperature. By operating at low DT, when the batch temperature rises slightly, heat evolution increases, but the delta T increases more significantly, thus removing the extra heat and driving the reaction temperature back to the target temperature.
Keith Dackson, Ph.D., Engineering Scientist
Henkel Technologies/Loctite Electronics, Olean, N.Y.We successfully scaled up a highly exothermic and "unusual" batch isomerization reaction from 1 liter in a calorimeter to a 30-gal. pilot-scale batch and from there to a 500-gal. full-scale reaction. We were able to abandon a $20-MM plant modification based on a continuous reactor and substituted a $5-MM (1980s $$) modification based on a couple of batch reactors. Learn From a scale up
I would proceed as follows:Run the reaction in a batch-heat-flow/heat-compensation calorimeter. This let's you run the reaction under conditions where the heat-transfer surface to reaction-volume ratio is very large. You can run the reaction in batch or semibatch mode; you can add the catalyst all at once, or add it slowly, i.e., you can explore heat evolution (and yields) as a function of time under various conditions. Since heat evolution is always some function of reaction rate, you're actually getting a real-time view of the reaction kinetics.Once you have these data, first determine how large a surface-to-volume ratio you need to prevent a runaway reaction. (Remember - batch reactions are always potential bombs, since during an upward temperature excursion, the heat transfer capability increases linearly with cooling media Add even more heat transfer area so that you can run at a relatively small Even if you only have a jacket, you must pump the cooling media around - so that it is essentially isothermal. The cooling loop acts as big flywheel. Cooling media temperature is adjusted by adding coolant to the pump-around loop and bleeding off an equivalent amount.Should the cooling media become too cold (e.g., during a process interruption), you need the capability of heating the pump around loop to get the reaction started again.Initially, when our ChEs tried to run the isomerization on a 10 gal. scale, they ended up with two runaways out a total of four reactions. And even for the successful ones, it was like balancing an egg on a pin.There was a real incentive to go batch,"it was much cheaper. I finally built my own heat-compensating calorimeter in one afternoon (a 1 liter resin kettle, submerged in a temperature-controlled bath, with an electric immersion heater in the reactor for temperature control). It was 1980, before commercial calorimeters were available. During the first run, we found that the reaction was not 1st order (as we had suspected). Here is what we observed: Initially, heat release was quite slow. Then, after about 30 min., the heat release started to pick up, but then diminished exponentially (as expected). It was that 30 min. phenomenon that caused the runaway reactions.There was obviously an "initiation" period, where an intermediate compound was formed, which then further reacted to complete the isomerization. We never determined the mechanism (it wasn't really important) because we now knew why we had the runaway reactions. We scaled up directly to a 30 gal. pilot-plant reactor, with a pump-around loop. We actually started out at a higher temperature, to get the reaction going, and as the heat release increased, we lowered the reaction temperature to maintain a constant heat evolution.On the second 30-gal. run, we only made a single temperature adjustment to the cooling loop,"and the reaction ran perfectly. When we then scaled up 500 gal. commercial batches (which required an external heat exchanger), we never had any problems with temperature control.
Gary Knapp, President GFK Consulting Ltd.,
San Clemente, Calif.Subject to limitations due to reactor volume or agitation requirements, use a high-heat-capacity inert such as tabular alumina spheres to dampen temperature swings. Tabular alumina spheres of 1/2 to 3/4-in. diameter would provide a significant heat sink, a high void fraction and would not significantly impede flow from the reactor at the end of a cycle.
Bill Norman, Technology Manager
Porocel Corp., [email protected]I think you meant solution polymerization. In the past, I have used an external cooler (heat exchanger) installed in the reactor circulation line. Depending on polymer properties and cleaning considerations, monomer injection can be on the inlet (preferred) or outlet of the cooler.It's the media's fault
Add external cooling
Monomer injection should be interlocked with high/low temperature on the reactor to prevent run-away exotherm and accumulation of unreacted monomer. Circulation pump and cooling should also be interlocked.
Robert Bryant II, Manufacturing Engineer
Valent USA, Walnut Creek, Calif.For a tight temperature control we often use a loop with a relative high-flow-capacity pump (about 5 to 10 times the vessel content per hour) and feed the loop with fresh cooling water over a PID-controlled three-way valve. This is a good and reliable solution in case the amount of heat to be removed gives a relatively small temperature rise in the cooling water. That's why this flow has to be as large as possible. The background of the story is that the heat buffer capacity of the cooling water is so large that the response time becomes also large. This gives the control loop the time to adjust the temperature before it runs away. I hope you can do something with this suggestion. However, the mixing action of the agitator is also a very important variable in this problem and in most cases the bottleneck.
Alan Ferraro, V.P. Engineering
Jongia Mixing/MPE Group USA, Pennsauken, N.J.The reader is right about PID control. You can get rapid response, but have too much overshooting and swings or you can control the overshooting and swings but have long process times and very sluggish response. Most exothermic reactions only allow a very narrow temperature band but require a rapid response to "catch" and control the reaction when it occurs. Allowing the exothermic reaction to get too hot or too cold can ruin the product batch. It is a significant issue with polymers.Lots of water flow buffers response time
Consider vacuum steam
We have a patented system that can control to +/- 1.8 Degrees F (1 Degrees C) and provide instant response to process fluctuations and temperature profiles. It is especially well-suited for exothermic reactions since it provides both heating and cooling capabilities. The heating system provides vacuum steam as low as 10 Degrees F. A pressure control valve uses proprietary control algorithms to allow rapid, measured response with minimal overshooting and swings. The cooling system uses an atomized water spray, which vaporizes under vacuum conditions to take advantage of the latent heat transfer at a much lower temperature (below 32 Degrees F if using brine solution). Product cooling times can be reduced by about 25%. Additionally, these systems provide energy and space savings when replacing hot water systems. By the way, did you know that vacuum steam has more BTU/lb. to transfer in latent heat? Just an added bonus.
Steve Kortheuer
TLV Corp., Charlotte N.C.Initially this sounds like the temperature setpoint (PID control) on the cooling water is too low. But I'm sure there is more to it than that. I suspect there is more than one process parameter that controls the reaction time.Vary the setpoint
Perhaps the process requires maintaining one temperature when in steady state but could be allowed to raise a given amount when the catalyst is added. If this is the case, the controller could switch to a higher setpoint when triggered that the catalyst is being added. The setpoint could go back to the initial value after a set time period. Some PID controllers can store more that one setpoint and switch them based on time or logic.For other situations, consider an over-ride controller. This consists of two PID controllers on one box that share an output (or set of outputs if both heating and cooling are controller). A simple rule set determines which PID controller had control of the output. Usually this is based on which controller output is calling for the lowest output, but this is configurable. Over-ride allows one output to be independently controlled by two parameters via two PID controllers.
Jim Overturf, VP of Marketing,
Eurotherm Inc., Leesburg, Va.The problem is that a tight temperature control is difficult to achieve by indirect cooling through a jacket of the vessel ," because of the high energy produced by the reaction, the heat to be removed is also high. The limitation to removing that heat will be mainly on the product side (not in the jacket).Improve the stirring
One simple way to improve the heat transfer is stirring. But in order to stir, the solution or suspension has to be diluted. Thus the concentration of active substance is lower and so too is conversion and reaction speed. If the suspension is particularly dense, the agitator will not move or, using a tougher, kneader type unit, the induced energy will be eventually even higher through mechanical dissipation.One appropriate method to overcome this problem is evaporative cooling. This means the operating pressure is adjusted so that one of the components in the reaction mixture (likely the solvent or suspension media) simply boils. You will need a closed design of the equipment and pressure control. The evaporated solvent can be recycled internally through a simple heat exchanger. You have to check if there is danger that one of the active substances of the reaction is stripped out and chose your solvent accordingly.
Daniel Witte, Senior Process & Sales Engineer,
List USA Inc., Charlotte, N.C.If a liquid is exposed to a pressure lower than its steam pressure, it starts evaporating and radiating heat through the developing vapors until the steam pressure is equal to the ambient pressure. In this process, the liquid cools down to the temperature corresponding to the prevailing pressure.Vacuum recirculation cooling
If the homogeneous mix of the product is exposed to underpressure, water will evaporate from the mix until the pressure of the water is equal to the interior pressure in the mixer. With the developing vapor, a heat stream is dissipated from the paste in the form of evaporative heat. At this point, the paste cools down to the temperature corresponding to the prevalent pressure according to the water vapor chart.With our vacuum cooling method, the cooling is generated in the product itself, independent from cooling surfaces (contact cooling) or from environmental conditions (evaporative cooling).A rotating mixing pan and rotary mixing tool keeps the product constantly moving, thus supporting the evaporation process.The temperature of the prepared product in the mixer is determined by the generated vacuum (underpressure), because pressure and temperature are related according to the water vapor chart. With a pre-selected defined pressure value, it is possible to automatically control the product temperature by means of the process controller.Occurrence of partial temperature differences in the product is impossible. The product quantity is entirely exposed to the vacuum, so that the water evaporates evenly throughout. The water vapor originated during the product cooling process is evacuated through a surface condenser, which consists of a bundle of thin-walled tubes with external circulation of cooling water. The developing condensate flows immediately back into the mixer, again being mixed homogeneously into the product. The processes of evaporation, condensation, and recirculation are occurring simultaneously during the cooling phase.
Nick Semitka
Eirich Machines, Gurnee, Ill.The problem does not lie in the method of control (PID in this case) or the tuning of the controllers. It is a heat transfer issue. Consider the enthalpy balance:DT, while heat generated increases exponentially with reaction temperature). If your reactor has insufficient jacket area, incorporate a pump-around loop to get sufficient heat exchange area.DT between cooling media and reactants. By doing this, the cooling media temperature essentially determines the reaction temperature. By operating at low DT, when the batch temperature rises slightly, heat evolution increases, but the delta T increases more significantly, thus removing the extra heat and driving the reaction temperature back to the target temperature.
Latest from Reaction & Synthesis
Latest from Reaction & Synthesis