More Related Content on Heat Tracing
Keeping equipment from getting too cold often is critical for operations and safety reasons. For instance, to prevent freeze expansion damage, plants in chilly areas generally use electricity or steam to heat trace water lines. While this addresses an obvious hazard, it also can pose less apparent pitfalls, as one plant discovered. That facility uses two parallel pumps to transfer water from a sump to a storage tank upstream of its treatment unit (Figure 1). Local climate conditions made freeze protection on the water line mandatory. The normal operating temperature of the line was 140°F. Standard practice was to wash the water line with an acid treatment on a scheduled basis. Over time, maintenance requirements on the pumps kept rising. Even with constant pump maintenance, their capacity seemed to keep dropping. Indeed, the sump level control valve (on the pump discharge) was wide open fairly rapidly — but pump capacity still was insufficient. Yet, field investigation showed the pumps’ discharge pressure met performance specifications. 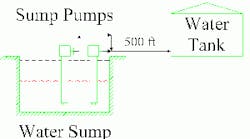
Figure 1. Getting into Hot Water: Tracing led to too hot a pipe, which
Figure 2. Which curve is Right? Using “good” data, two methods
Latest from Reliability & Maintenance
Latest from Reliability & Maintenance

