Question from January's Chemical Processing
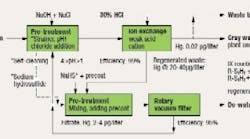
We are formulating a plan for collecting mercury from the blowdown in our scrubbers. Our on-site contractor has provided a basic design for consideration involving ion exchange (IX) and sulfide vacuum filtration (Figure 1). The proposal is for continuous removal of mercury using the maximum concentration range of 1.5–2.0 μg/l as a design basis (EPA studies). The proposal includes a reference to separation processes developed by the U.S. Department of Defense and Department of Energy to handle mercury waste. Using sludges of heavy metals, a 99% recovery can be assumed for the ion exchange, according to the studies. The regenerated waste produced by the IX unit will be treated with a sulfide and run through a vacuum filter: filtrate will then be reprocessed through the IX unit while sludge is collected for disposal. The vendor’s sales engineer claims that it may be possible to turn a profit by distilling mercury from the solid waste collected after de-watering. Are there any flaws in the proposal? How should we proceed?
Over-optimist
The concentration of Hg is fairly low so the efficiencies of the ion exchanger will probably be lower than the EPA report indicates. In EPA/625/R-97/004, the reported efficiencies were sometimes as low as 95% for concentrations in the 200–70,000 μg/liter range. The starting concentration of mercury in the scrubber blowdown is only 1.5–2.0 μg/liter. The efficiency of the ion exchange membranes will probably drop; mercury will have to compete against other more agile cations such as sodium, potassium and magnesium.
With a new process, the efficiencies must be thoroughly checked out. Perhaps a bench test could be carried out using average samples from the scrubber. These should be representative of the cations expected on a daily basis.
The vendor's claims seem dubious. Separating the mercury from the other solid waste in the filter cake will probably be expensive and lead to many environmental headaches.
Other problems with this process are: Will all the Hg0 in the flue gases be converted to Hg+ or Hg2+ in the scrubber? How will heat of reaction affect the efficiency of the ion exchanger(s)?
John Corn, consultant
Hilliard, Ohio
If the process is less efficient than expected, it may be necessary to consider other technology such as a granulated activated carbon (GAC) or a second precipitation step for the product stream from the IX unit. Another approach might be an absorbent called Thiol-SAMMS (self-assembled monolayers on mesoporous supports). One of these methods could then polish the water before returning it to the plant water supply as gray water. Or, possibly, in the case of Thio-SAMMS, it might be considered a replacement for ion exchange. An alternative unit operation could be a second or third ion exchanger.
— Dirk Willard, senior editor
Activated carbon may be a better choice
At one time, two guys in the Cleveland suburbs were providing all sorts of coatings on activated carbon, but I've lost track of them. It is certainly a lot cheaper, and more straightforward, than the ion exchanger, but one would need a deep bed.
About 40 years ago, I worked on an activated carbon bed for Polaroid's battery plant. But, if I recall correctly, the washdown was treated with boron hydride (can't remember why), plus Zn(OH)2 to create the Zn/Hg amalgam, plus a flocculating polymer — and all went through a plate and frame, with very fine cloth. However, I have no idea of the removal efficiency, but the effluent ran down to a reservoir for raw drinking water.
Most people tell me they send any amalgam back to a smelter, but I've never checked on that. If you're pushing large volumes through a filter, then you are correct: The operating expense of vacuum filters is probably less expensive than a plant and frame filter.
One thing not mentioned is the temperature. Since Hg melts around -38°C, and has 1 mm Hg vapor pressure at 126°C, I'm assuming very little Hg is vaporized at STP. Having said that, even the small number of electron volts extant, could cause big problems, over time, but I haven't seen EPA's recent emission standards. Thus, removal efficiencies may need to be 99.9999%, which is tough to do in mass transfer.
Tom Murphy, CEO
Puritrol, Inc., Centerville, Mass.
Try an absorbent
A better solution than an ion exchanger might be a mercury absorbent. We market such an absorbent. This material is available in powder, granular, or pellet form. The powder form may be used as a filter aid in the filtration step or in a separate step. The granular form is used in packed column, as you would, GAC or IX.
The sludge from the filter is a wet cake (40-50 % moisture), which is typical for pressure or vacuum filters. Removing additional water would require some kind of drying step.
To see an illutration of the solutions, click here.
Matt A. Omofoma, Ph.D., manager
World Minerals, Inc., Lompoc, Calif.
May's Puzzler
A decanter serves a distillation column used to separate water from solvents. Recovered solvents are then burned in a thermal oxidizer (TOX). The decanter separates the heavy organics, such as carbon tetrachloride, from water. The tank diameter is 48 in. and has a volume of 1,000 gallons. During start-up, it is noticed that the minute the pump begins to fill the tank the high level switch, 90° from the tank inlet, trips the pump off (Figure 2). What can be done quickly to eliminate this problem and keep the overflow safeguard?
Figure 2. Splashing causes high level switch to trip — killing pump prematurely.
Send us your comments, suggestions or solutions for this question by April 2, 2007. We’ll include as many of them as possible in the May 2007 issue and all on CP.com. Send visuals — a sketch is fine. E-mail us at [email protected] or mail to ProcessPuzzler, Chemical Processing, 555 W. Pierce Rd., Suite 301, Itasca, IL 60143. Fax: (630) 467-1120. Please include your name, title, location and company affiliation in the response.
And, of course, if you have a process problem you’d like to pose to our readers, send it along and we’ll be pleased to consider it for publication.