Researchers at Washington State University (WSU), Pullman, Wash., have developed a nickel-based catalyst that weakens the methane molecule’s hydrogen-carbon bond, allowing it to break at much lower temperatures. The catalyst could lead to significant energy savings and reduced pollution, they say.
The research, led by Jean-Sabin McEwen, assistant professor, and Su Ha, associate professor, in the school of chemical engineering and bioengineering, revealed that adding a small amount of carbon within the nickel-based catalyst creates nickel carbide, which generates a positive electrical field. They have built a numerical model of the reaction and currently are testing it experimentally. An article in Angewandte Chemie has more details on their work.
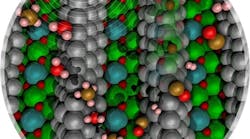
Figure 1. Methane carbon-hydrogen bond activation over nickel clusters (silver spheres) is promoted by low concentrations of surface carbon (brown spheres within the nickel cluster) or by a positive electric field. Source: Washington State University.
“We want to experimentally validate our computational results, which show evidence that a small amount of carbon would change the electronic state of the Ni nanoparticles that are supported over a metal oxide. As the charge state of Ni changes from a neutral species to a partially positively charged Ni ion, we want to [show] that it is easier to activate methane when Ni is partially positively charged,” notes McEwen.
“We have already begun preliminary experiments, but have been unable to observe the catalytic enhancement as predicted by theory so far. … to see a catalytic enhancement, we need a low concentration of carbon, but carbon deposits too quickly on our supported Ni cluster under our reaction conditions, which leads to a severe catalytic deactivation due to coking. We are currently working on ways to retard the coking rate so as to be able to monitor the catalytic activity when a low concentration of carbon is deposited on Ni. For example, one way to do this is to dope the Ni catalyst with small amounts of Sn,” says Ha.
[callToAction ]
In previous work, the researchers were only able to apply a moderate external electric field. They are now modifying the catalyst bed to increase the magnitude of applied electric field while only applying an external bias of 200 V. “With such an effort, we believe that we can enhance the positive electric field effect and increase the methane conversion. In this way, we will be able to lower the required operating temperature for the reaction,” explains McEwen.
After initial studies are completed, the team will more carefully investigate the long-term stability of the catalyst. In terms of catalyst poisoning, the researchers are aware that too much C acts as a poison. “We do not plan to carburize the catalyst to that extent. Instead, we need to understand the initial behavior of the catalyst when a very small concentration of C is added to it. We will accomplish this by doping the catalyst with Sn, for example, so as to avoid its poisoning.”
“Our published theoretical work is a very interesting fundamental investigation, but its result applies to only a small window of time during the actual reaction if the catalyst is not further modified. Presently, understanding this phenomenon may be important when designing a catalytic reactor, particularly during the start-up process. The challenge we see, … is that while the presence of a few carbon atoms may indeed improve catalytic ability, this improvement will inevitably lead to further carbon deposition if the catalyst is not altered somehow to avoid its deactivation,” elaborates McEwen.
If such a system can be designed, it could prove to be a very valuable new catalyst technology that could lead to huge cost savings by lowering the operating temperature and steam requirement in a chemical plant, they believe.