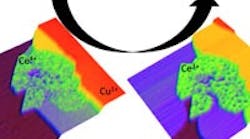
SCANNING TUNNELING MICROSCOPY
Microscope image shows a cerium-oxide and copper catalyst (CeOx-Cu) used in the transformation of carbon dioxide and hydrogen gases to methanol and water. Source: Brookhaven National Laboratory.
“Developing an effective catalyst for synthesizing methanol from CO
2 could greatly expand the use of this abundant gas as an economical feedstock,” says Brookhaven chemist Jose Rodriguez, who led the research. Such a catalyst could help mitigate greenhouse gas by capturing CO
2 emitted from methanol-powered combustion engines and fuel cells, and recycling it to synthesize new fuel.The team explored a catalyst composed of copper and ceria (cerium-oxide) nanoparticles, sometimes also mixed with titania. In previous studies, the metaloxide nanoparticle catalysts demonstrated exceptional reactivity in a variety of reactions; highly reactive sites formed where the interfaces of the two types of nanoparticles met.To study the reactivity of dual-particle catalytic systems in converting the carbon dioxide to methanol, scientists used in-situ imaging under reaction conditions and chemical “fingerprinting” techniques. These techniques allowed the scientists to peer into the dynamic evolution of a variety of catalysts as they operated in real time. They also used computational modeling to provide a molecular description of the methanol synthesis mechanism.The results showed that the metal component of the catalysts alone could not carry out all the chemical steps necessary to produce methanol. The most effective binding and activation of CO
2 occurred at the interfaces between metal and oxide nanoparticles.“The key active sites for the chemical transformations involved atoms from the metal [copper] and oxide [ceria or ceria/titania] phases,” explains Jesus Graciani, a chemist from the University of Seville. The resulting catalyst converts CO
2 to methanol more than a thousand times faster than plain copper particles, and almost 90 times faster than a common copper/zinc-oxide catalyst currently in industrial use.More details appear in a recent issue of the journal
Science.The next step, to begin this year, involves preparing and characterizing high-surface-area powders that can be used efficiently in industrial applications. Rodriguez notes it’s unclear how long this will take and the key challenge will be to create a stable copper-ceria interface in the powder catalyst. To do this, the team will examine the possible effects of ppm levels of sulfur on the catalytic activity, and test for stability under different CO
2/H
2 reactant ratios for long periods of time.Once this work is done, the team will conduct studies on a larger laboratory or pilot-plant scale.