Scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, Upton, N.Y., have discovered a low-cost, sustainable electrocatalyst that efficiently generates hydrogen gas for fuel. The combination of nickel, molybdenum and nitride in a nanosheet structure rivals the performance of platinum — which is the most effective catalyst for splitting off the hydrogen, but extremely expensive.
Two-dimensional Nanosheet
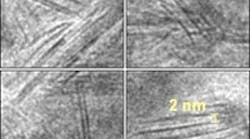
Figure 1. This magnified image reveals the dark, straight lines of the nanosheet structure found in the nickel-molybdenum-nitride catalyst. Source: Brookhaven National Laboratory. While nickel could be used in place of platinum, it lacks comparable electron density. So, to enhance its reactivity, the scientists introduced metallic molybdenum. But the performance levels of platinum were still unmatched. "We needed to introduce another element to alter the electronic states of the nickel-molybdenum, and we knew that nitrogen had been used for bulk materials, or objects larger than one micrometer," says research associate Wei-Fu Chen. "But this was difficult for nanoscale materials, with dimensions measuring billionths of a meter."The team infused the nickel-molybdenum with nitrogen by subjecting it to a high-temperature ammonia environment. This unexpectedly transformed the particles into two-dimensional nanosheets, resulting in a catalyst that nearly matches platinum's high-density reactivity (Figure 1)."Despite the fact that metal nitrides have been extensively used, this is the first example of one forming a nanosheet," Chen says. "Nitrogen made a huge difference — it expanded the lattice of nickel-molybdenum, increased its electron density, made an electronic structure approaching that of noble metals, and prevented corrosion."An article in Angewandte Chemie International Edition details the process.The next step, already underway, is to optimize the nickel-molybdenum (Ni/MO) ratio in the catalyst. "The one we published is the best so far. We have not tuned the optimal mole ratio of Ni and Mo for the current catalyst, so we may find it to further improve the HER [hydrogen evolution reaction] activity. There are, of course, other choices of metals that combine one metal that binds hydrogen weakly and one that binds it strongly so that the net binding would be similar to that of Pt. We will explore some of these to see if they make nanosheets with the desired properties," says James Muckerman, the senior chemist who led the project.In addition, the team will test the catalyst's durability in electrolyzer/photochemical cell systems. The researchers hope to improve the activity and durability of the catalyst. "We expect the lifetime of the catalyst will be longer than 20,000 hrs … The catalyst is extremely stable, even in strong acid, at negative applied potentials, but, as we indicated in the Angewandte Chemie article, it starts to become unstable at potentials more positive that +0.8 V…. Its possible usefulness would be in the HER in the context of water electrolyzers or solar photoelectrichemical cells," explains Muckerman.Other factors that might affect long-term performance include dissolution or oxidation, as well as the segregation of the catalyst surface. So far, there's been no susceptibility to poisoning, but further exploration is needed, says Muckerman.The team hopes to scale up sometime this year, and currently is exploring ways to make 500-g batches. "The synthesis method is simple, and the scale-up of catalyst production should be straightforward.""The important key that makes our catalyst more active and stable compared with other NiMo catalysts is 'nitridation' to incorporate nitrogen in NiMo. The treatment gave: (i) high corrosion resistance; (ii) a unique nanostructure, i.e., "nanosheets, and as a consequence, high surface areas are attained; and (iii) an increase in HER activity by changing electronic structures of both Ni and Mo toward that of Pt.," Muckerman concludes.